Novel 2-substituted-5-(4-chloro-2-phenoxy)phenyl-1,3,4-oxadiazole derivatives, ligands of GABAA/benzodiazepine receptor complex
Design, synthesis, radioligand binding assay, and pharmacological evaluation
DOI:
https://doi.org/10.17179/excli2022-5639Keywords:
benzodiazepine, binding assay, 1,3,4-oxadiazole, docking, hypnotic, anticonvulsantAbstract
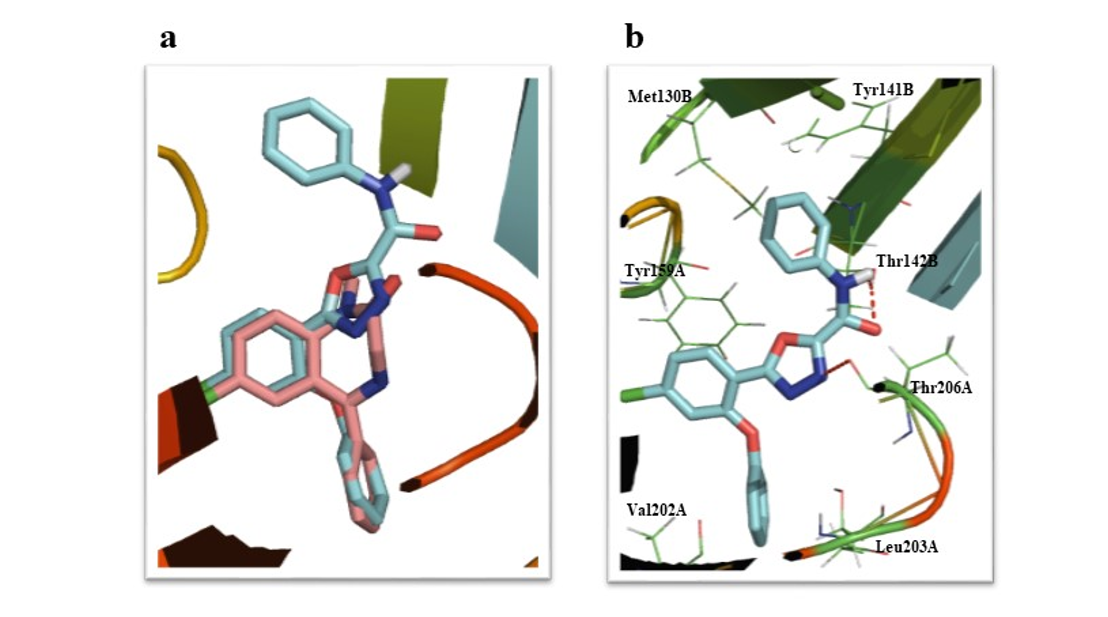
Agonists of Benzodiazepine (BZD) receptor are exhaustively used in the control of muscle spasms, seizure, anxiety, and insomnia. BZDs have some unwanted effects; therefore, the development of new BZD receptor agonists with better efficacy and fewer unwanted effects is one of the subjects of interest. In this study, based on the pharmacophore/receptor model of the BZD binding site of GABAA receptors, a series of new 2-substituted-5-(4-chloro-2-phenoxy)phenyl-1,3,4-oxadiazole derivatives (6a-f) were designed. Energy minima conformers of the designed compounds and diazepam were well matched in conformational analysis and showed proper interaction with the BZD-binding site of the GABAA receptor model (α1β2ϒ2) in docking studies. The designed compounds were synthesized in acceptable yield and evaluated for their in vitro affinity to the benzodiazepine receptor of rat brains by radioligand receptor binding assay. The results demonstrated that the affinities of most of the novel compounds were even higher than diazepam. The novel compound 6a with the best affinity in radioligand receptor binding assay (Ki=0.44 nM and IC50= 0.73±0.17 nM) had considerable hypnotic activity and weak anticonvulsant and anxiolytic effects with no negative effect on memory in animal models. Flumazenil as a selective benzodiazepine receptor antagonist was able to prevent hypnotic and anticonvulsant effects of 6a indicating the role of BZD receptors in these effects.
Downloads
Additional Files
Published
How to Cite
License
Copyright (c) 2023 Elham Rezaee, Fatemeh Ahmadi, Mahsa Shabaninia , Mona Khoramjouy, Zahra Azizi Farsani, Soraya Shahhosseini, Sayyed Abbas Tabatabai, Mehrdad Faizi

This work is licensed under a Creative Commons Attribution 4.0 International License.
Authors who publish in this journal agree to the following terms:
- The authors keep the copyright and grant the journal the right of first publication under the terms of the Creative Commons Attribution license, CC BY 4.0. This licencse permits unrestricted use, distribution and reproduction in any medium, provided that the original work is properly cited.
- The use of general descriptive names, trade names, trademarks, and so forth in this publication, even if not specifically identified, does not imply that these names are not protected by the relevant laws and regulations.
- Because the advice and information in this journal are believed to be true and accurate at the time of publication, neither the authors, the editors, nor the publisher accept any legal responsibility for any errors or omissions presented in the publication. The publisher makes no guarantee, express or implied, with respect to the material contained herein.
- The authors can enter into additional contracts for the non-exclusive distribution of the journal's published version by citing the initial publication in this journal (e.g. publishing in an institutional repository or in a book).





