Editorial
Recent studies on flavonoids and their antioxidant activities
Soo Cheon Chae1, Jai-Heon Lee2, Sang Un Park3
1Department of Horticultural Science, College of Industrial Sciences, Kongju National University, 1 Daehoe-ri, Yesan-kun, Chungnam, 340-720, Korea
2Department of Genetic Engineering, Dong-A University, Busan 604-714, Korea
3Department of Crop Science, College of Agriculture and Life Sciences, Chungnam National University, 99 Daehangno, Yuseong-gu, Daejeon, 305-764, Korea
EXCLI J 2013;12:Doc226
Flavonoids are widely distributed plant secondary metabolites with various metabolic functions. They are ubiquitous in fruits and vegetables that are regularly consumed by humans. These natural compounds are categorized by their chemical structure into 6 major subgroups as follows: chalcones, flavones, flavonols, flavandiols, anthocyanins, and proanthocyanidins or condensed tannins (Winkel-Shirley, 2001[27]; Falcone Ferreyra et al., 2012[7]). More than 6000 different flavonoids have been identified, and this number is certain to increase as more researches are conducted on them (Ferrer et al., 2008[8]).
Flavonoids have attracted considerable interest because of their potentially beneficial effects in humans; they have been reported to have antiviral, antiallergic, antiplatelet, antiinflammatory, antitumor, and antioxidant activities (Izzi et al., 2012[13]; Kay et al., 2012[14]). Many investigations have focused on these health-promoting effects and antioxidant activities of flavonoids, particularly their role in the chemoprevention of cancer (Gonzalez-Paramas et al., 2011[11]; Galleano et al., 2012[9]). We have reviewed the most recent studies on flavonoids and their antioxidant activities (Table 1(Tab. 1)). (References in Table 1: Corradini et al., 2011[3]; Morales et al., 2012[20]; Gong et al., 2010[10]; Liu et al., 2012[17]; Seelinger et al., 2008[23]; Calderón-Montaño et al., 2011[2]; Wang et al., 2010[26]; Margina et al., 2012[18]; Liu et al., 2012[16]; Waisundara et al., 2011[25]; Marković et al., 2009[19]; Hyun et al., 2010[12]; Devi et al., 2010[5]; Verdan et al., 2011[24]; Li et al., 2011[15]; Yang et al., 2012[28]; Annadurai et al., 2012[1]; Rossato et al., 2011[22]; Park et al., 2010[21]; Duchnowicz et al., 2012[6]; de Pascual-Teresa et al., 2010[4]).
Notes
Jai-Heon Lee and Sang Un Park (Department of Crop Science, College of Agriculture and Life Sciences, Chungnam National University, 99 Daehangno, Yuseong-gu, Daejeon, 305-764, Korea; phone: +82-42-821-5730; email: supark@cnu.ac.kr ) contributed equally as corresponding authors.
Acknowledgements
This work was carried out with the support of the "Cooperative Research Program for Agriculture Science & Technology Development (Project No. PJ906938)" Rural Development Administration, Republic of Korea.
References
1.
Annadurai T, Muralidharan AR, Joseph T, Hsu MJ, Thomas PA, Geraldine P. Antihyperglycemic and antioxidant effects of a flavanone, naringenin, in streptozotocin-nicotinamide-induced experimental diabetic rats. J Physiol Biochem. 2012;68:307-18. 2.
Calderón-Montaño JM, Burgos-Morón E, Pérez-Guerrero C, López-Lázaro M. A review on the dietary flavonoid kaempferol. Mini Rev Med Chem. 2011;11:298-344.3.
Corradini E, Foglia P, Giansanti P, Gubbiotti R, Samperi R, Lagana A. Flavonoids: chemical properties and analytical methodologies of identification and quantitation in foods and plants. Nat Prod Res. 2011;25:469-95.4.
de Pascual-Teresa S, Moreno DA, García-Viguera C. Flavanols and anthocyanins in cardiovascular health: a review of current evidence. Int J Mol Sci. 2010;11:1679-703.5.
Devi VG, Rooban BN, Sasikala V, Sahasranamam V, Abraham A. Isorhamnetin-3-glucoside alleviates oxidative stress and opacification in selenite cataract in vitro. Toxicol In Vitro. 2010;24:1662-9. 6.
Duchnowicz P, Broncel M, Podsędek A, Koter-Michalak M. Hypolipidemic and antioxidant effects of hydroxycinnamic acids, quercetin, and cyanidin 3-glucoside in hypercholesterolemic erythrocytes (in vitro study). Eur J Nutr. 2012;51:435-43. 7.
Falcone Ferreyra ML, Rius SP, Casati P. Flavonoids: biosynthesis, biological functions, and biotechnological applications. Front Plant Sci. 2012;3:222.8.
Ferrer J, Austin M, Stewart CJ, Noel J. Structure and function of enzymes involved in the biosynthesis of phenylpropanoids. Plant Physiol Biochem. 2008;46:356–70.9.
Galleano M, Calabro V, Prince PD, Litterio MC, Piotrkowski B, Vazquez-Prieto MA, et al. Flavonoids and metabolic syndrome. Ann N Y Acad Sci. 2012;1259:87-94.10.
Gong G, Qin Y, Huang W, Zhou S, Yang X, Li D. Rutin inhibits hydrogen peroxide-induced apoptosis through regulating reactive oxygen species mediated mitochondrial dysfunction pathway in human umbilical vein endothelial cells. Eur J Pharmacol. 2010;628:27-35. 11.
Gonzalez-Paramas AM, Santos-Buelga C, Duenas M, Gonzalez-Manzano S. Analysis of flavonoids in foods and biological samples. Mini Rev Med Chem. 2011;11:1239-55.12.
Hyun J, Woo Y, Hwang DS, Jo G, Eom S, Lee Y, et al. Relationships between structures of hydroxyflavones and their antioxidative effects. Bioorg Med Chem Lett. 2010;20:5510-3. 13.
Izzi V, Masuelli L, Tresoldi I, Sacchetti P, Modesti A, Galvano F, et al. The effects of dietary flavonoids on the regulation of redox inflammatory networks. Front Biosci. 2012;17:2396-418.14.
Kay CD, Hooper L, Kroon PA, Rimm EB, Cassidy A. Relative impact of flavonoid composition, dose and structure on vascular function: A systematic review of randomised controlled trials of flavonoid-rich food products. Mol Nutr Food Res. 2012;56:1605-16.15.
Li R, Yuan C, Dong C, Shuang S, Choi MM. In vivo antioxidative effect of isoquercitrin on cadmium-induced oxidative damage to mouse liver and kidney. Naunyn Schmiedebergs Arch Pharmacol. 2011;383:437-45. 16.
Liu B, Jian Z, Li Q, Li K, Wang Z, Liu L, et al. Baicalein protects Human melanocytes from H(2)O(2)-induced apoptosis via inhibiting mitochondria-dependent caspase activation and the p38 MAPK pathway. Free Radic Biol Med. 2012;53:183-93.17.
Liu H, Zhang L, Lu S. Evaluation of antioxidant and immunity activities of quercetin in isoproterenol-treated rats. Molecules. 2012;17:4281-91.18.
Margina D, Ilie M, Manda G, Neagoe I, Mocanu M, Ionescu D, et al. Quercetin and epigallocatechin gallate effects on the cell membranes biophysical properties correlate with their antioxidant potential. Gen Physiol Biophys. 2012;31:47-55.19.
Marković ZS, Mentus SV, Dimitrić Marković JM. Electrochemical and density functional theory study on the reactivity of fisetin and its radicals: implications on in vitro antioxidant activity. J Phys Chem A. 2009;113:14170-9.20.
Morales J, Günther G, Zanocco AL, Lemp E. Singlet oxygen reactions with flavonoids. A theoretical - experimental study. PLoS One. 2012;7:e40548.21.
Park CE, Yun H, Lee EB, Min BI, Bae H, Choe W, et al. The antioxidant effects of genistein are associated with AMP-activated protein kinase activation and PTEN induction in prostate cancer cells. J Med Food. 2010;13:815-20.22.
Rossato MF, Trevisan G, Walker CI, Klafke JZ, de Oliveira AP, Villarinho JG, et al. Eriodictyol: a flavonoid antagonist of the TRPV1 receptor with antioxidant activity. Biochem Pharmacol. 2011;81:544-51. 23.
Seelinger G, Merfort I, Schempp CM. Anti-oxidant, anti-inflammatory and anti-allergic activities of luteolin. Planta Med. 2008;74:1667-77.24.
Verdan AM, Wang HC, García CR, Henry WP, Brumaghim JL. Iron binding of 3-hydroxychromone, 5-hydroxychromone, and sulfonated morin: Implications for the antioxidant activity of flavonols with competing metal binding sites. J Inorg Biochem. 2011;105:1314-22. 25.
Waisundara VY, Siu SY, Hsu A, Huang D, Tan BK. Baicalin upregulates the genetic expression of antioxidant enzymes in Type-2 diabetic Goto-Kakizaki rats. Life Sci. 2011;88:1016-25. 26.
Wang ZH, Ah Kang K, Zhang R, Piao MJ, Jo SH, Kim JS, et al. Myricetin suppresses oxidative stress-induced cell damage via both direct and indirect antioxidant action. Environ Toxicol Pharmacol. 2010;29:12-8.27.
Winkel-Shirley B. Flavonoid biosynthesis. a colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiol. 2001;126:485–93.28.
Yang HL, Chen SC, Senthil Kumar KJ, Yu KN, Lee Chao PD, Tsai SY, et al. Antioxidant and anti-inflammatory potential of hesperetin metabolites obtained from hesperetin-administered rat serum: an ex vivo approach. J Agric Food Chem. 2012;60:522-32.
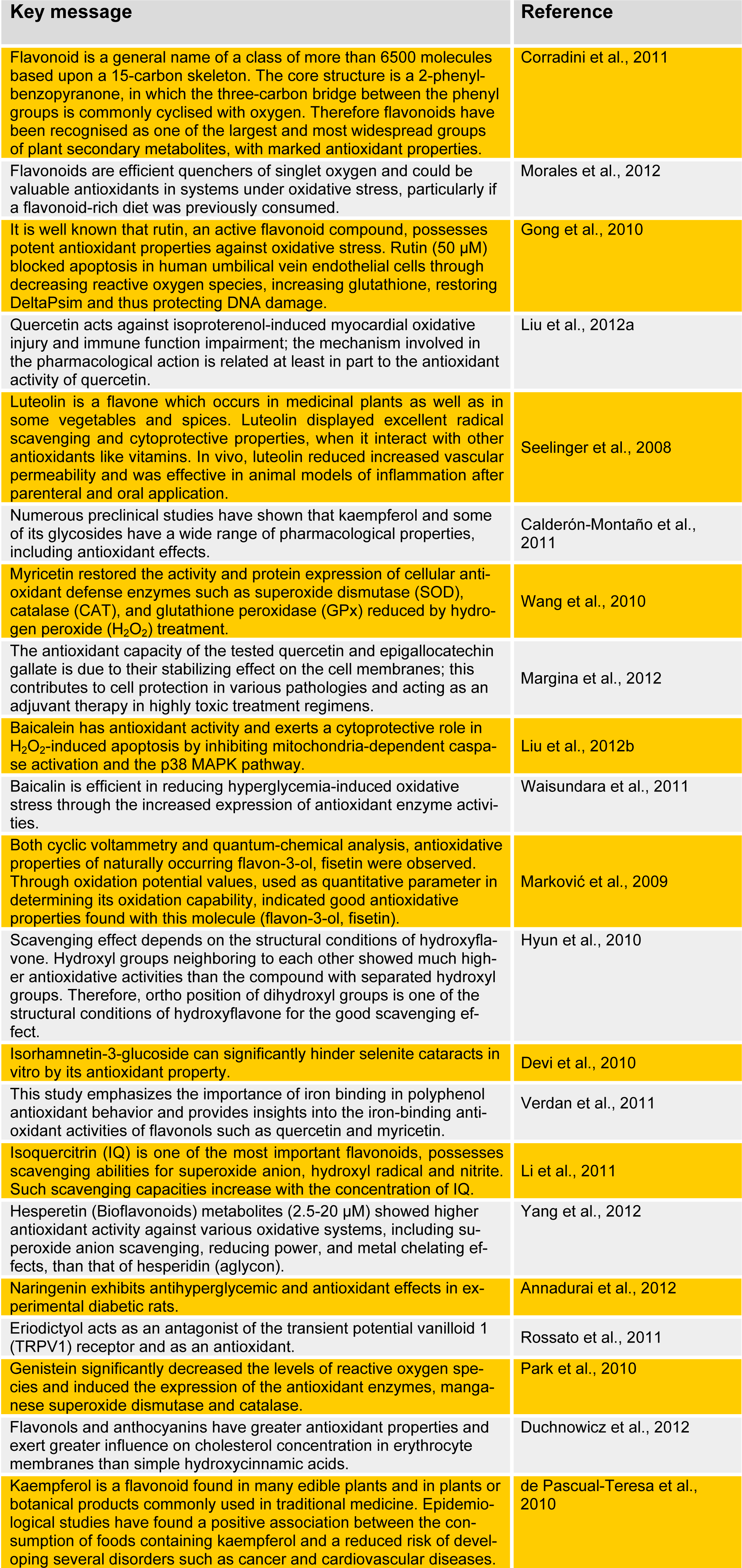
Table 1: Recent studies on flavonoid compounds and their antioxidant activities
