Research article
Ethanolic extract of Commiphora mukul gum resin attenuates streptozotocin-induced alterations in carbohydrate and lipid metabolism in rats
B. Ramesh1, R. Karuna2, S. Sreenivasa Reddy3, G. Sudhakara3, D. Saralakumari3[*]
1Department of Biochemistry, Sri Venkateswara University, Tirupati - 517502, Andhra Pradesh, India2Department of Internal Medicine, University of Nebraska Medical Centre, Omaha, NE, USA
3Department of Biochemistry, Sri Krishnadevaraya University, Anantapur - 515003, Andhra Pradesh, India
EXCLI J 2013;12:Doc556
Abstract
The purpose of this study was to investigate the effects of Commiphora mukul gum resin ethanolic extract (CMEEt) administration against altered activities of key enzymes of carbohydrate metabolism, lipid metabolism and changes in glycogen content (liver and muscle) and lipids (liver and heart) in streptozotocin (STZ) induced insulin deficient diabetic Wistar albino rats. Diabetes was induced by intraperitoneal injection of STZ (55 mg/kg body wt) to male Wistar rats. The animals were divided into four groups: Control (C), control-treated (C+CM), diabetic (D) and diabetic-treated group (D+CM). Diabetic-treated and control-treated rats were treated with C. mukul gum resin ethanolic extract (CMEEt) in 2 ml distilled water, orally (200 mg/kg body weight/day for 60 days). At the end of the experimental period, biochemical parameters related to carbohydrate and lipid metabolism were assayed. The significant enhancement in tissue lipids (heart and liver) total cholesterol, triglycerides, phospholipids and free fatty acids of diabetic rats were nearer to normalized in diabetic treated rats (D+CM). Alterations in the activities of enzymes of glucose metabolism (hexokinase, phosphofructokinase, pyruvate kinase, and glucose-6-phosphatase, fructose-1,6-bisphosphatase and glucose-6-phosphate dehydrogenase) and lipid metabolism (fatty acid synthase, malic enzyme and lipoprotein lipase) as observed in diabetic (D) rats were prevented with CMEEt administration. In conclusion, our findings indicate improvement of glucose and lipid metabolisms in STZ induced diabetic rats by treatment with Commiphora mukul and suggest that the plant can be used as an adjuvant for the prevention and/or management of insulin deficiency and disorder related to it.
Keywords: C. mukul, diabetes, glycolysis, gluconeogenesis, lipogenesis, tissue lipids
Introduction
Diabetes mellitus is a metabolic disorder of multiple aetiology characterized by chronic hyperglycemia with disturbances of carbohydrate, fat and protein metabolism resulting from defects in insulin secretion, insulin action or both (Kaleem et al., 2008[27]). Globally, the estimated incidence of diabetes and projection for year 2030, as given by International Diabetes Federation (IDF) is 350 million (Thounaojam et al., 2010[51]). The rapid increase in the incidence of type-1 diabetes strongly suggests that the action of the environment on susceptibility genes contributes to the evolving epidemiology of type-1 diabetes (Gillespie, 2006[22]).
Liver is an insulin dependent tissue, which plays a pivotal role in glucose and lipid homeostasis and is severely affected during diabetes mellitus. It has been shown to decrease the activities of enzymes in the glycolytic and pentose phosphate pathways, increased activities of gluconeogenesis and glycogenolytic pathways (Mc Anuff et al., 2005[34]). Medicinal plants play an important role in the management of diabetes mellitus especially in developing countries where resources are meager. Many studies have confirmed the benefits of medicinal plants with hypoglycemic effects in the management of diabetes mellitus. The effects of these plants may delay the development of diabetic complications and correct the metabolic abnormalities. Moreover, during the past few years some of the bioactive drugs isolated from hypoglycemic plants showed antidiabetic activity with more efficacy than oral hypoglycemic agents used in clinical therapy (Bnouham et al., 2006[5]). Herbal remedies are apparently effective with minimal or no side effects in chemical experience and are of relatively low costs as compared to oral synthetic hypoglycemic agents (Gupta et al., 2005[24]).
Commiphora mukul (synonym C. whighitti) (family Burseraceae) commonly called as gum guggulu is highly valued in Ayurveda an Indian system of medicine practiced in India, Bangladesh and Pakistan. It is a large thorny shrub or a small tree, with spiny, ascending branches. The gum resin extract of C. mukul tree has been used in Ayurvedic medicine for more than 2000 years to treat a variety of ailments like obesity, lipid disorders, rheumatoid arthritis, bone fracture, cardiovascular disorder disease (Chander et al., 2003[11]). Traditional (India) uses of C. mukul (CM) are for its anti-inflammatory, antispasmodic, carminative, emmenagogue, hypoglycemic, alternative, antiseptic, and astringent, a thyroid stimulant, anthelminitic, antihyperlipidemia, antidiabetic and antioxidant properties (Ramesh et al., 2011[38]). It is an important herb used in the treatment of several degenerative disorders in modern medicine too and established as a hypolipidemic drugs (Ulbricht et al., 2005[52]). Ayurvedic medicines containing gum guggul often contain guggul in their names, such as in Shunthi-guggul, and Yogaraja guggul. All the formations used for traditional Ayurvedic treatments for obesity contained gum guggul among its herbal ingredients. Two stereo isomers, E- and Z-guggulsterone (cis- and trans- 4, 17(20)-pregnadiene-3, 16-dione, respectively), are the most important constituents studied in detail for their therapeutic potential from C. mukul. Various studies have been conducted to understand and illustrate the mechanism of action and potential of guggulsterone as a therapeutic agent using synthetic E and Z isomers (Burris et al., 2005[8]; Ding and Staudinger, 2005[15]; Ichikawa and Aggarwal, 2006[25]).
Earlier work in our laboratory has shown that alcoholic extract of C.mukul gum resin has significant antihyperglycemic and antioxidant activities in STZ induced diabetic rats and fructose fed insulin resistant Wistar rats (Ramesh et al., 2011[38], Ramesh and Saralakumari, 2012[40]). Guggulsterone, the bioactive constituent of guggul, has been identified as an antagonist of the nuclear farnesoid X receptor (FXR) (Urizar et al., 2002[53]) a key transcriptional regulator for the maintenance of cholesterol and bile acid homeostasis (Cai and Boyer, 2006[9]). Although numerous reports are available on the hypolipidemic nature and its plausible relation with type II diabetes treatment, in place of natural guggulsterone its synthetic counterparts have been used in most of the cases. The synthetic products mainly consist of either E- or Z-forms instead of the combination of them, as they exist naturally in guggul lipids along with other components as mentioned and recommended in traditional Ayurvedic literature.
Material and Methods
Chemicals
Acetyl Co-A, malonyl Co-A, pyruvate kinase, streptozotocin, lactate dehydrogenase and glucose-6-phosphate dehydrogenase were obtained from the Sigma Chemicals Co. St. Louis, Mo, USA. All other chemicals and solvents were of analytical grade and procured from Sisco Research Laboratories (P) Ltd., Mumbai, India.
Plant material
Ethanolic extract of Commiphora mukul gum resin (CMEE; brown, dry powder with Lot No. L5111031) was obtained from the manufacturers and exporters of herbal extracts, Ms Plantex Pvt. Ltd., Vijayawada, Andhra Pradesh, India. Procedure followed by the firm for the preparation of extract is as follows: the plant was identified by Dr. K. Narasimha Reddy, Taxonomist, Laila impex R & D Center, Vijayawada. The collected plant sample (resin) was washed thoroughly with tap water, dried at room temperature away from sun light, cut into small pieces, and then powdered. Ethanolic extract was prepared by cold maceration of gum resin powder in ethanol for 7 days. The extract was filtered, concentrated under reduced pressure and finally dried in a vacuum desiccator. Herb-to-product ratio was 8:1. A voucher specimen has been deposited in the Department of Biochemistry, Sri Krishnadevaraya University, Anantapur, under number DSK-CM-09. The extract was stored at 0-4 °C and dissolved in water just before use.
Animals
Nine week old male albino Wistar rats of body weight 160 - 180 g were procured from Sri Raghavendra enterprises (Bangalore, India), acclimatized for 7 days to our animal house, and maintained at standard conditions of temperature and relative humidity, with a 12 h light/dark cycle. Water and commercial rat feed were provided ad libitum. The current work was carried out with a prior permission from our institutional animal ethical committee (Regd. No.470/01/a/CPCSEA, dt 24th Aug 2001). Diabetes was induced in overnight fasted rats by a single intraperitonial injection of freshly prepared STZ solution (55 mg/Kg/ body weight, in ice cold 0.1M citrate buffer, pH 4.5 in a volume of 0.1 ml per rat). After 72 h of STZ administration, the plasma glucose level of each rat was determined for confirmation of diabetes. Rats with plasma glucose level above 250 mg/dl were considered as diabetic and were used in the experiment.
Experimental design
In the present experiment, a total of 32 rats (16 diabetic rats; 16 normal rats) were used. The rats were divided into four groups of 8 each:
- Control (C);
- Control rats treated with C. mukul (C+CM);
- diabetic (D); and
- diabetic rats treated with C. mukul (D+CM).
Diabetic treated group and Control treated group received an ethanol extract of the Gum resin of C. mukul (2 ml of 5 % Tween-80) by orogastric tube at a dose of 200 mg/kg body weight for 60 days, whereas, 2 ml of distilled water was administered to control and diabetic rats. Based on preliminary experiment on dose-dependent antihyperglycemic effect of CMEEt, a dose less than 200 mg/kg body eight was not expected to be effective in rats (Ramesh et al., 2011[38]). At the end of experimental period of 60 days, animals were sacrificed by cervical decapitation. Liver, kidney, heart and visceral adipose tissues were collected. The extraction of lipids from liver and heart were carried out according to the procedure of Folch et al. (1957[19]). Total cholesterol, triglycerides (Span Diagnostic kit, Surath, India), free fatty acids (Duncombe, 1963[18]) and phospholipids (Connerty et al., 1961[12]) were analyzed. Protein content in the tissue homogenates was estimated by the method of Lowry et al. (1951[33]).
Glycogen estimation
Hepatic and muscle glycogen content were determined by using anthrone reagent as adapted by (Carroll et al., 1956[10])
Enzyme assays
10 % liver homogenate was prepared in ice cold 0.1 M Tris-HCl buffer, pH 7.4, centrifuged at 12,000 rpm for 45 min in Sigma laboratory centrifuge 3K18 model, rotor no. 12150. The clear supernatant thus obtained with the cytosolic fraction was used for the assay of hexokinase (Brandstrup et al., 1957[6]), phosphofructokinase (Sadava et al., 1997[43]), pyruvate kinase (Sadava et al., 1997[43]), glucose-6-phosphatase (King, 1965[29]), fructose-1,6-bisphosphatase (Sadava et al., 1997[43]), glycogen phosphorylase (Sutherland, 1955[49]), glucose-6- phosphate dehydrogenase (Beutler, 1975[4]), fructokinase (Adelman et al., 1966[2]) and malic enzyme (Geer et al., 1980[20]). For the fatty acid synthetase, the tissue was homogenized at 4 °C in 0.1 M potassium phosphate buffer, pH 8.0. The homogenate was centrifuged at 30, 000 rpm at 4 °C for 30 min and the clear supernatant collected was used for the assay of fatty acid synthetase (Gibson and Hubbard, 1960[21]). Assay of lipoprotein lipase in adipose tissue was done following the method of Shirai and Jackson (1982[46]).
Statistical analysis
Data were expressed as the mean ± SE for the number, n = 8 of animals in the group as indicated in (Tables 1-3(Tab. 1)(Tab. 2)(Tab. 3)). The data were subjected to statistical analysis by Duncan's Multiple Range (DMR) (Duncan, 1955[17]) test. Values of p < 0.05 were considered statistically significant.
Results
Effect of CMEEt on glycogen content in liver and skeletal muscle
The glycogen content of liver and skeletal muscle of experimental group is presented in Figure 1(Fig. 1). The D-Group showed significantly decreased glycogen content in liver (61.2 %) and muscle (68.3 %) when compared to control group. Significant increase in the glycogen content of liver (60 %) and muscle (67.24 %) by C. mukul administration in DT group was observed when compared to the D-group which reached normal levels. C. mukul treated control rats (C+CM) showed no significant variation in the glycogen content of liver and muscle when compared to control rats.
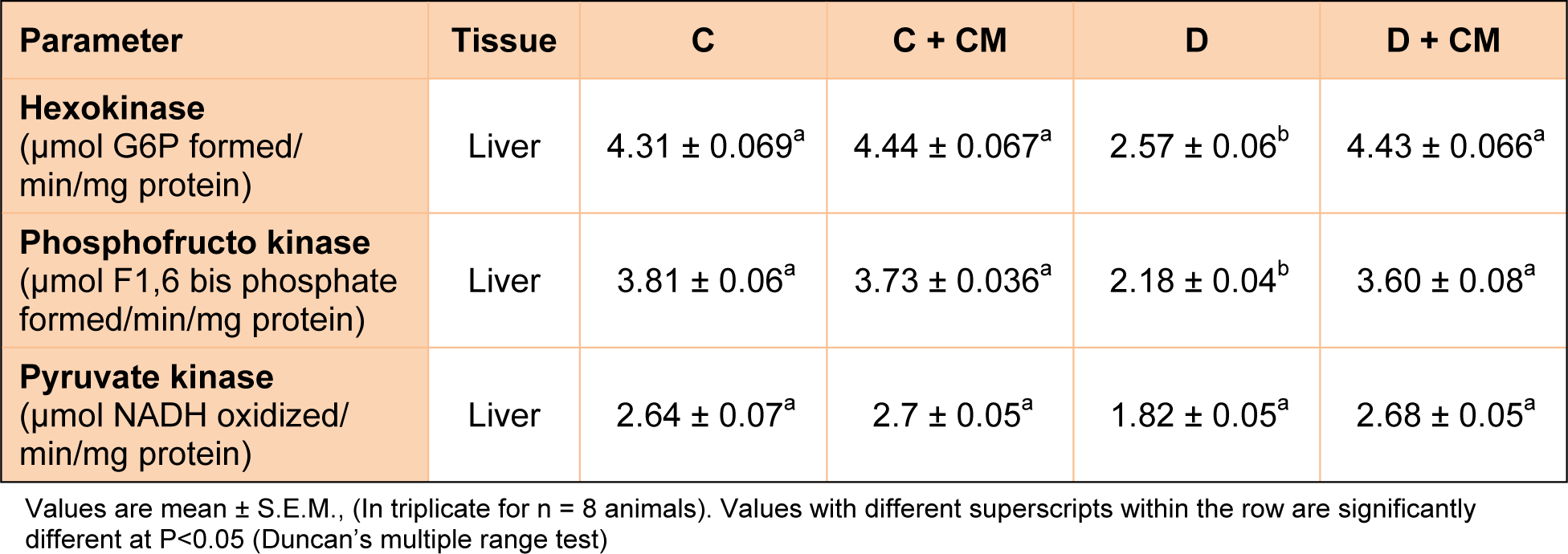
Effect of C. mukul on the activities of hexokinase, phosphofructokinase, pyruvate kinase, fructokinase and glucose-6-phosphate dehydrogenase
Table 1(Tab. 1) and Figure 2(Fig. 2) and 3(Fig. 3) show the changes in the activities of HK, PFK, PK, FK and glucose-6-phosphate dehydrogenase in liver of control and experimental rats. The activities of HK, PFK, PK, PFK and glucose-6-phosphate dehydrogenase were significantly decreased in STZ induced diabetic rats when compared with control rats. Oral administration of C. mukul gum resin ethanol extract to diabetic rats significantly increased the activities of these hepatic enzymes. The administration of C. mukul gum resin ethanol extract to normal rats (controls) resulted in no significant changes.
Effect of C. mukul on the activities of F1, 6-bisphosphatase, glucose 6-phosphatase and glycogen phosphorylase
Table 2(Tab. 2) depicts the activities of fructose 1,6-bisphosphatase, glucose-6-phosphatase and glycogen phosphorylase in control and experimental rats. The activities of fructose 1, 6 bisphosphatase, glucose-6-phosphatase and glycogen phosphorylase were significantly increased in diabetic rats. Oral administration of C. mukul gum resin ethanol extract to diabetic rats reversed the changes in the activities of these hepatic enzymes. The administration of C. mukul gum resin ethanol extract to control rats produced no significant changes in these enzymatic activities when compared with control rats.
Effect of C. mukul on tissue lipids in STZ diabetic rats
Group D showed Table 3(Tab. 3) significantly increased liver and heart total cholesterol, triglycerides, free fatty acid and phospholipids when compared with control rats. Administration of C. mukul gum resin ethanol extract in STZ induced diabetic rats significantly restored the liver and heart total cholesterol, triglycerides, free fatty acids, and phospholipids almost equal to control rats.
Discussion
The main objective of the present study is to determine whether Commiphora mukul gum resin, implicated as a hypoglycemic agent, modulates key enzymes involved in glucose metabolism in two peripheral tissues during its course of action. We report here that C. mukul gum resin treated to STZ-induced diabetic rats modulate key glucose metabolizing enzymes in both liver and kidney, resulting in normal blood glucose homeostasis. STZ-injection activation of G6PDH and decreased glucose production by depression of key gluconeogenic and glycogenolytic enzymes. In the present study the significantly decreased activities of these glycolytic enzymes observed in the liver and skeletal muscle of STZ diabetic rats indicated decreased operation of glycolysis under diabetic condition in the insulin dependent tissue (skeletal muscle) and insulin independent tissue (liver). Decreased HK activity also indicates decreased availability of glucose-6-phosphate for HMP shunt operation under diabetic condition. A similar trend of decreased glycolytic enzyme activities was reported in various tissues of diabetic animals (Rathi et al., 2002[41]).
Lowered hepatic G6PDH activity was observed in STZ-diabetic rats. Our results are in accordance with the earlier reports (Bugdayci et al., 2006[7], Karuna et al., 2010[28]). This decrease may be considered as an adaptive phenomenon by which the supply of NADPH for aldose reductase is cut down. Reduced activity of this enzyme in diabetic state shows that glucose does not enter into pentose phosphate pathway to a greater extent. G6PDH activity and NADPH/NADP ratio vary inversely in relation to blood glucose concentration. Studies of Díaz-Flores et al. (2006) indicated that decrease in hepatic G6PDH activity is dependent on the severity of hyperglycemia. The decreased activity of G6PDH also indicates that the HMP shunt activity is unable to meet the requirement of cellular NADPH for the enzymes that continuously maintain Hb and GSH in their reduced states. The decreased activity of this enzyme further indicates the accumulation of glucose-6-phosphate which is a potent glycosylating agent that causes GSH depletion and thereby boosts glycation (Jain, 1998[26]) and it may also promote the final step of gluconeogenesis (G6Pase). C. mukul administration induced moderate reversal of this effect in D + CM- rats. Insulin is reported to stimulate oxidation of glucose by increasing the activation of G6PDH in dose dependent manner (Wagle et al., 1998[54]).
In our study, administration of C. mukul significantly increased the activity of G6PDH in diabetic state. It provides hydrogen, which binds to NADP+ and produces NADPH and enhances the synthesis of fats from carbohydrates, i.e, lipogenesis (Abdel-Rahim et al., 1992[1]), finally the plasma glucose levels were decreased. Previous studies show that Umbelliferone, a phenolic compound produces the same effect in STZ-diabetic rats (Ramesh and Pugalendi, 2006[39]). High level of total cholesterol is one of the major factors for coronary heart diseases and it is well known that hyperlipidemia and the incidence of atherosclerosis is increased in diabetes (Tan et al., 2005[50]). The liver and some other tissues participate in the uptake, oxidation and metabolic conversion of free fatty acids, synthesis of cholesterol and phospholipids and secretion of specific classes of plasma lipoprotein.
Liver plays an important role in buffering the postprandial hyperglycemia and is involved in synthesis of glycogen. Diabetes mellitus is known to impair the normal capacity of the liver to synthesize glycogen (Sirag, 2009[47]). Synthase phosphatase activates glycogen synthase, resulting in glycogenesis, and this activation appears to be defective in STZ-induced diabetic rats (Kirana and Srinivas, 2008[30]). Diabetic rats treated with CMEEt had liver glycogen brought back to near normal levels, which could be due to increased secretion of insulin, which enhances glycogenesis.
Hepatic lipid accumulation may be a result of one or several of the following factors: increased delivery of dietary fatty acids or fatty acids from adipose tissue to the liver, increased de nova synthesis of fatty acids in the liver, decreased rate of hepatic fatty acid oxidation, or decreased rate in the exit of fatty acid from the liver in the form of TG. Delivery of fatty acids to the liver appears to be the most potent mechanism for hepatic lipid accumulation (Donnelly et al., 2005[16]). The lipid lowering activity of the resin of C.mukul has been well documented in various hyperlipaemic experiment animals (Sharma et al., 2009[45]) and guggulsterone was used as positive control to assesses the hyperlipaemic activity of other chemical compound (Kumari and Augusti, 2007[31]). Several studies have shown that guggulsterone lowers cholesterol levels by lowering the LDL cholesterol but increasing the HDL cholesterol. Fatty acid synthase plays a central role in de novo lipogenesis in animals by catalyzing all the reactions in conversation of acetyl COA and malonyl CoA to palmitate. Malic enzymes play a key role in generation of NADPH for lipogenesis. The activates of hepatic malic enzyme correlate closely with the rate of lipid biosynthesis (Revilla et al., 1987[42]). Lipoprotein lipase is an enzyme responsible for the hydrolysis of triglycerides from plasma lipoproteins and its activity is influenced by nutritional and hormonal status and by environmental conditions (Merkel et al., 2002[35]). Adipose tissue LPL is the enzyme that allows the entry of lipoprotein packaged fatty acids into adipose tissue for storage (Appel et al., 1992[3]). Excess of fatty acids in serum produced by the streptozotocin-induced diabetes promotes conversion of excess fatty acids into phospholipids and cholesterol in liver. These two substances along with excess triglycerides formed at the same time in liver may be discharged into blood in the form of lipoproteins. The abnormal high concentration of serum lipids in the diabetic subject is mainly due to increase in the mobilisation of free fatty acids from the peripheral fat depots, since insulin inhibits the hormone sensitive lipase. Hypercholesterolemia and hypertriglyceridemia have been reported to occur in streptozotocin diabetic rats (Pushparaj et al., 2000[37]; Sharma et al., 1996[45]) and significant increase observed in our experiment was in accordance to these studies. The marked hyperlipidaemia that characterize the diabetic state may therefore, be regarded as a consequence of the uninhibited actions of lipolytic hormones on the fat depots (Goodman and Gilman, 1985[23])
The present studies show that the C. mukul gum resin ethanol extract possesses definite hypotriglyceridemic and antiatherogenic properties in STZ diabetic rats after 60 days of treatment. The repeated administration of C. mukul extract for a period of 60 days resulted in a significant decrease in lipid parameters of various tissues when compared to the diabetic control. Many studies have shown that the increase in fatty acid delivery to the liver leads to increased triglyceride synthesis accompanied by increases in VLDL secretion (Sharma et al., 2009[44]).
Conclusion
From the above findings, we conclude that C. mukul administration significantly increased insulin secretion and normalized the deranged carbohydrate metabolism and lipid metabolism in diabetic rats by enhancing glucose utilization and decreasing hepatic glucose production, thereby exhibiting significant antidiabetic effects. Further studies are needed to explore the molecular mechanisms in altering hormone secretion.
Conflict of interest
We declare that we have no conflict of interest. We wish to disclose that the study was carried out as part of Ph.D dissertation work of Dr. B. Ramesh, and no funds were received either from University or Govt. of India.
References
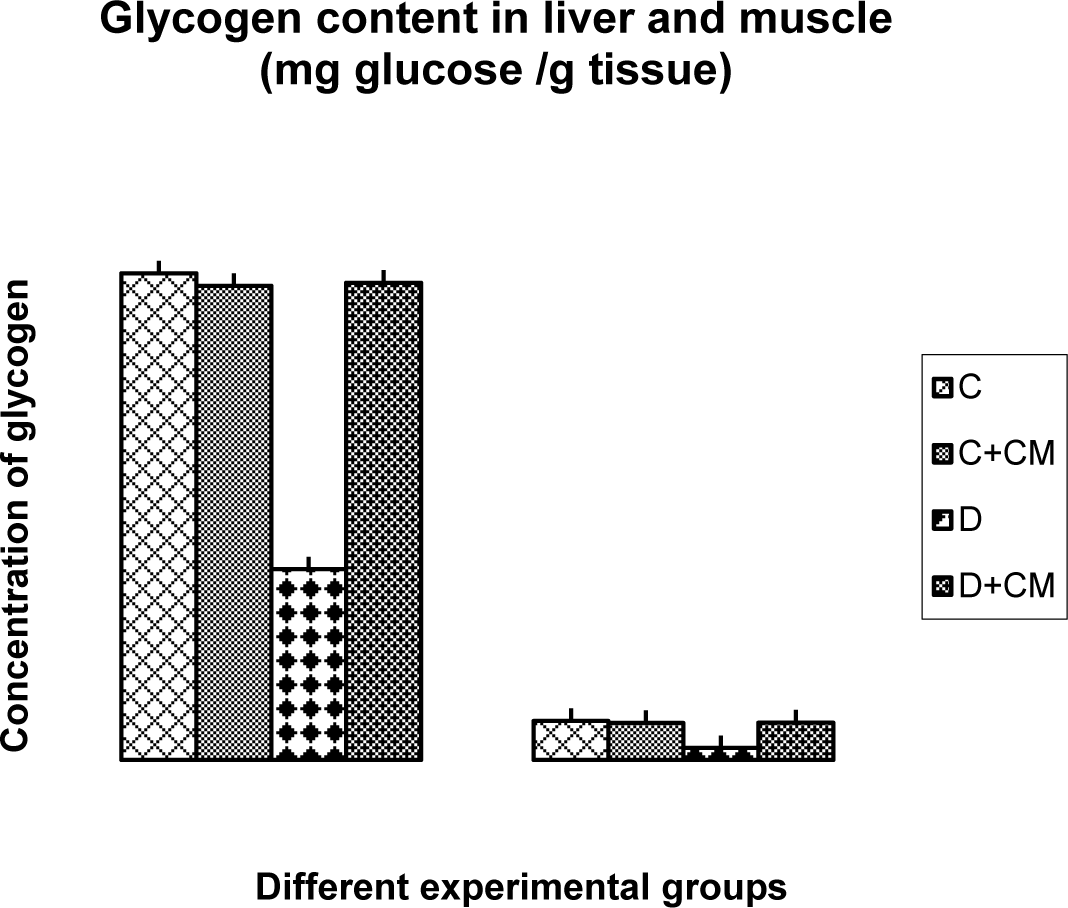
Figure 1: Change in glycogen content in liver and muscle of CON, CON+CMEE, D and D+CM groups during the 60 days experimental period. Values are ± S.E., (In triplicate for n = 8 animals).
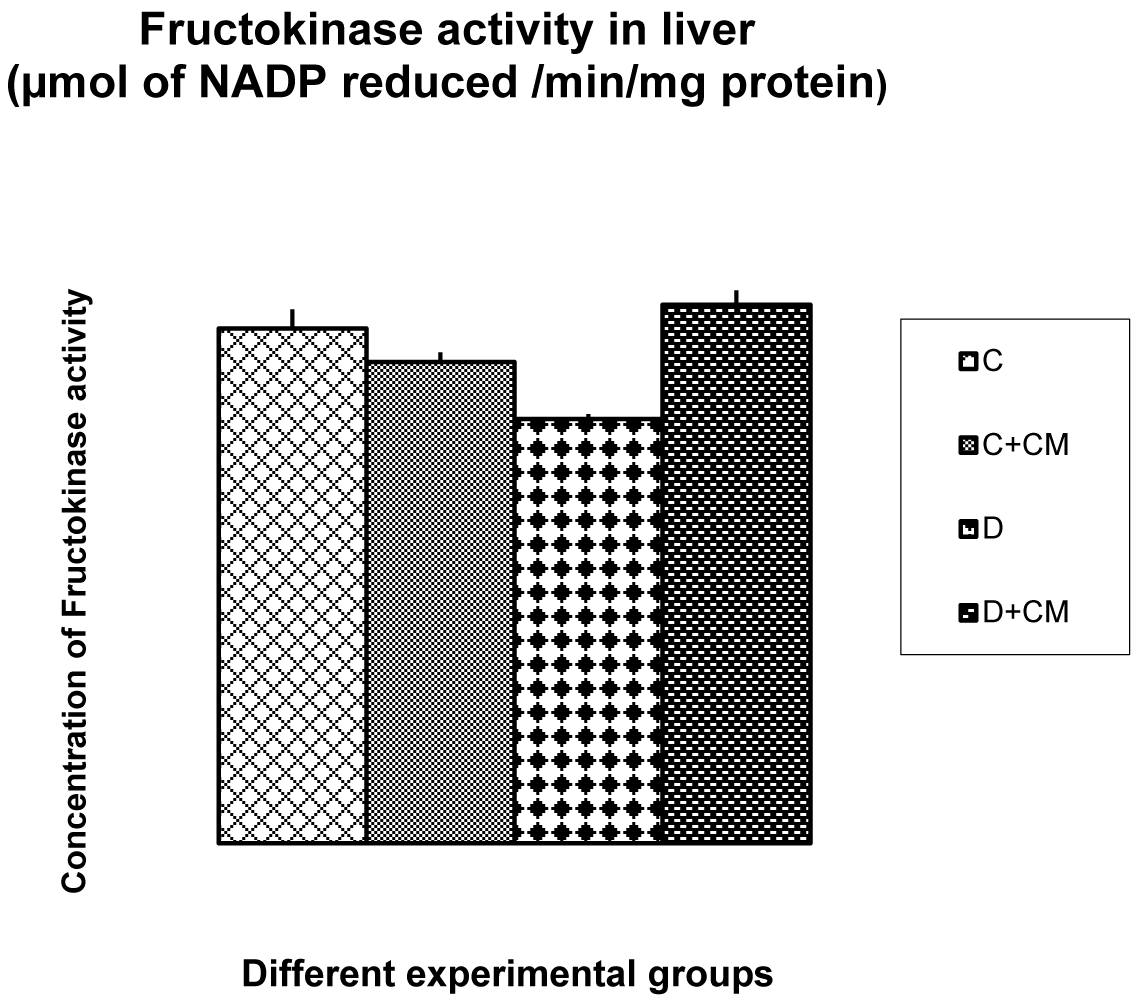
Figure 2: Change in fructokinase activity in liver of CON, CON+CMEE, D and D+CM groups during the 60 days experimental period. Values are ± S.E., (In triplicate for n = 8 animals)
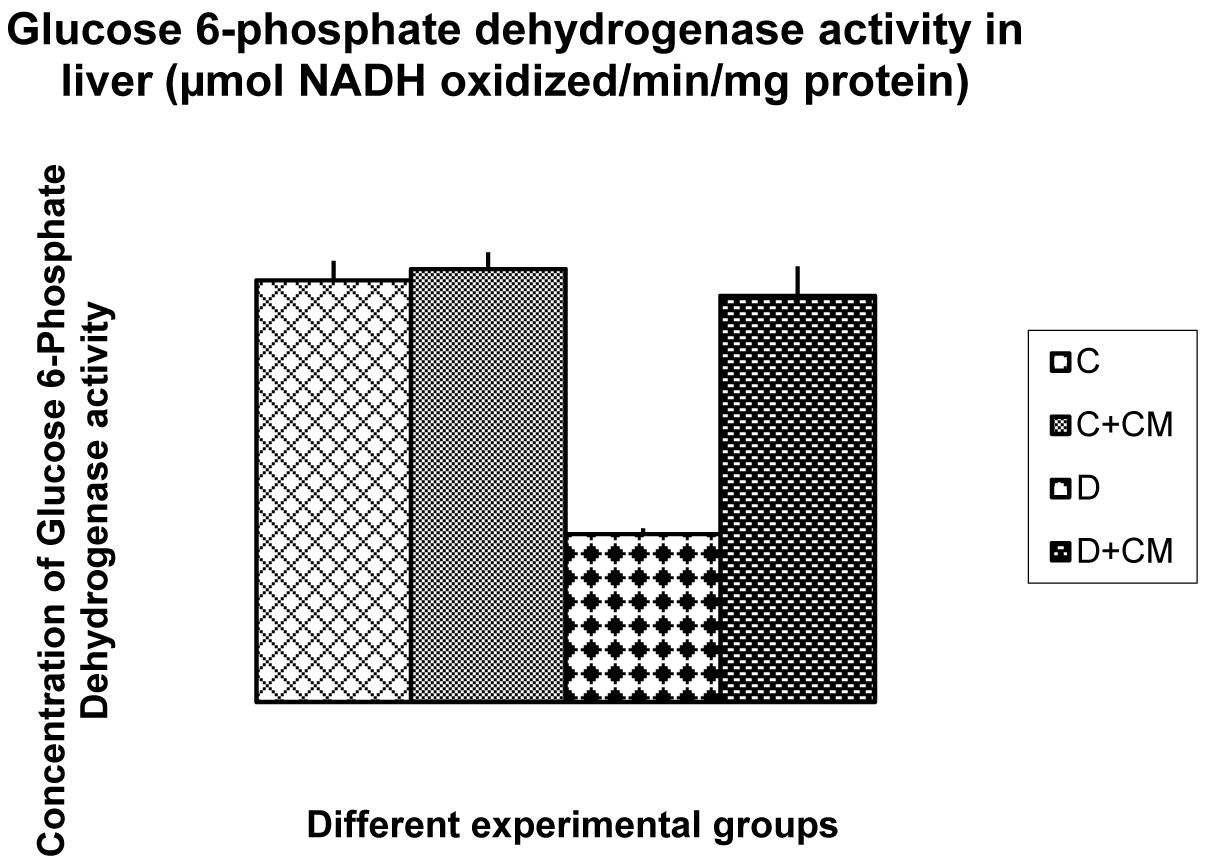
Figure 3: Change in glucose-6, phosphate dehydrogenase activity in liver of CON, CON+CMEE, D and D+CM groups during the 60 days experimental period. Values are ± S.E., (n = 8 animals)
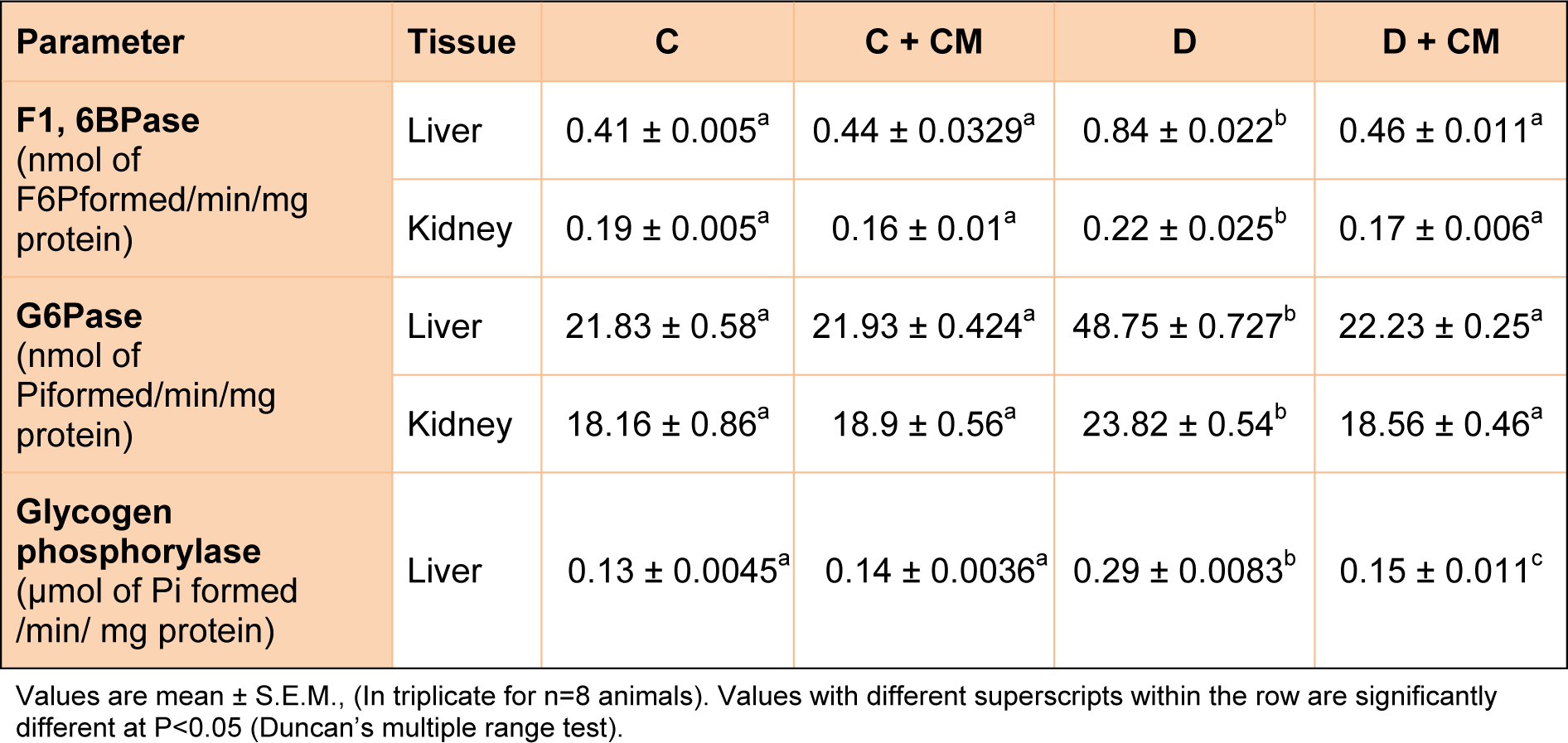
Table 2: Effect of C. mukul treatment on activity of gluconeogenic enzymes in liver and kidney of STZ diabetic rats.
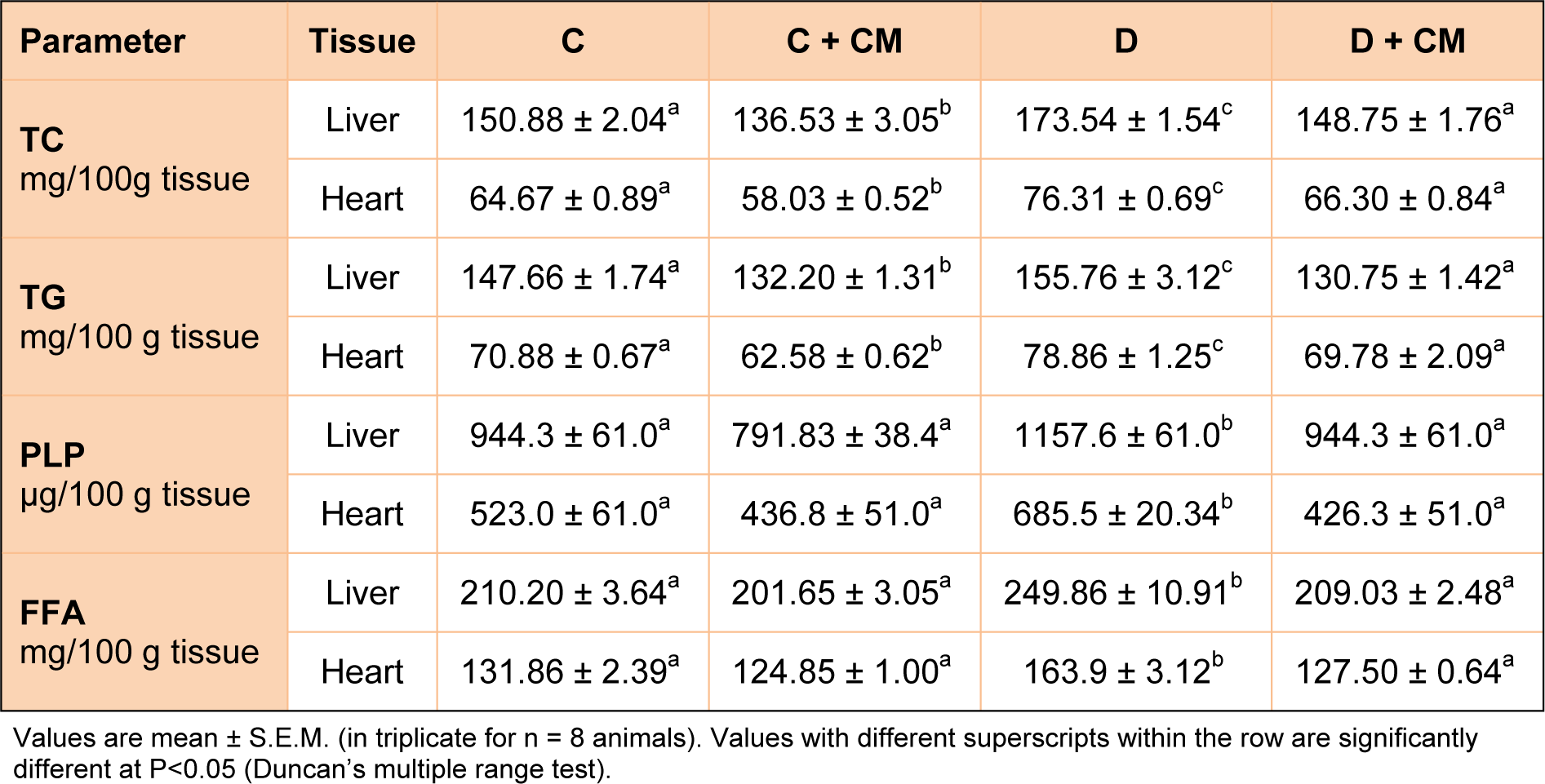
Table 3: Effect of C. mukul treatment on hepatic and cardiac tissue lipids in control and experimental rats
[*] Corresponding Author:
Prof. D. Saralakumari, Department of Biochemistry, Sri Krishnadevaraya University, Anantapur-515 003, India; Tel: +91-08554-255879; Fax: +91-08554-255805, eMail: skumari1@yahoo.co.in