Research article
The protective effects of pomelo extract (Citrus grandis L. Osbeck) against fructose-mediated protein oxidation and glycation
Natarin Caengprasath1,2, Sathaporn Ngamukote2,3, Kittana Mäkynen2,3, Sirichai Adisakwattana3[*],2
1Program in Food and Nutrition, Department of Nutrition and Dietetics, Faculty of Allied Health Sciences, Chulalongkorn University, Bangkok, 10330, Thailand2The Medical Food Research and Development Center, Department of Nutrition and Dietetics, Faculty of Allied Health Sciences, Chulalongkorn University, Bangkok, 10330, Thailand
3Research Group of Herbal Medicine for Prevention and Therapeutic of Metabolic Diseases, Chulalongkorn University, Bangkok, 10330, Thailand
EXCLI J 2013;12:Doc491
Abstract
Chronic hyperglycemia induces non-enzymatic protein glycation, which plays an important role in the development of diabetic complications. Immense efforts have been made to determine effective antiglycation compounds from natural products. Pomelo has shown beneficial effects for human health. The objective of this study was to determine the antiglycation effect of pomelo extract against fructose-mediated protein oxidation and glycation. Our results showed that the pomelo extract (0.25 - 2.00 mg/mL) significantly inhibited the overall formation of advanced glycation end products (AGEs) in a concentration-dependent manner. The pomelo extract markedly decreased the level of fructosamine, which is directly associated with reduction in formation of AGEs and Nε-(carboxymethyl)lysine (CML). In addition, the pomelo extract inhibited protein oxidation through its ability to prevent the loss of thiol groups and reduced protein carbonyl formation. We characterized the active components in the pomelo extract by using high-performance liquid chromatography (HPLC), which showed that the pomelo extract contained naringin (11.90 ± 0.21 mg/g dried extract), hesperidin (12.04 ± 0.12 mg/g dried extract), neohesperidin (25.4 ± 0.12 mg/g dried extract), and naringenin (9.20 ± 0.19 mg/g dried extract). Our findings could provide a new insight into the antiglycation properties of the extract of the naturally occurring fruit pomelo for preventing AGE-mediated diabetic complications.
Keywords: pomelo, flavonoids, glycation, HPLC
Introduction
Glycation is a non-enzymatic reaction that occurs between a carbonyl group of a sugar and an amino group of an amino acid, which leads to formation of reversible structure or a Schiff's base (Brownlee, 1995[8]). Subsequently, the initial Schiff's base undergoes rearrangements to from Amadori products, which induce further oxidation to generate dicarbonyl compounds to form cross-linking fluorescent (e.g. pentosidine) and non-fluorescent adducts (e.g. Nε-(carboxymethyl)lysine [CML]) called as advanced glycation end products (AGEs). Recent studies indicate that the formation and accumulation of AGEs in the tissues have been implicated in the progression of diabetic complications such as retinopathy, nephropathy, and neuropathy (Ahmed, 2005[4]; Schmidt and Stern, 2000[27]). Nowadays, antiglycation agents have attracted a lot of attention for alleviating diabetic complications (Wu et al., 2011[34]). A representative antiglycation drug is aminoguanidine (AG), which acts by blocking cross-link formation by interacting with post-Amadori reactive intermediates to prevent the formation of AGEs from dicarbonyl precursors (Thornalley, 2003[32]). However, previous studies have shown that AG has toxic effects in diabetes patients, such as flu-like symptoms, gastrointestinal problems and anemia (Bolton et al., 2004[7]; Thornalley, 2003[32]). Therefore, attention has also been focused on the antiglycation properties of phytochemicals present in fruits and vegetables (Adisakwattana et al., 2010[2]; Dearlove et al., 2008[11]; Wu et al., 2009[36]). Natural AGE inhibitors may have promising therapeutic potential in delaying the onset and preventing aging and diabetic complications.
Pomelo (Citrus grandis L.), which belongs to the family Rutaceae, is one of the most widely cultivated crops in Southeast Asia. In the past, the pulp of pomelo has been used as an appetizer, antitoxic, cardiac stimulant, and stomach tonic (Arias and Ramón-Laca, 2005[6]). The major flavonoids of pomelo are neohesperidin, hesperidin, naringenin, and naringin, which are present in high amounts in fruit juice (Kanes et al., 1993[14]; Kawaii et al., 1999[15]; Xu et al., 2008[37]). Recently, a number of studies on pomelo extract have reported the favorable antioxidant properties through free radical-scavenging effects in vitro (Chung, 2000[9]; Jayaprakasha et al., 2008[13]; Lim et al., 2006[18]). In addition, the pomelo extract reduces the reactive oxygen species in hydrogen peroxide (H2O2)-treated HepG2 cells (Lim et al., 2006[18]). However, to date, no study has reported the antiglycation activity of pomelo. In the present study, we clarified the effect of the pomelo extract against fructose-mediated non-enzymatic glycation. In addition, we examined the inhibitory effect of pomelo extract on oxidation-dependent damages induced by fructose to bovine serum albumin (BSA). This study will underline the importance of pomelo extract in prevention of hyperglycemia-mediated protein modification.
Materials and Methods
Chemicals
Naringin, hesperidin, neohesperidin, naringenin, neohesperidin dihydrochalcone, hesperitin, BSA (fraction V), AG hydrochloride, 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB), guanidine hydrochloride, nitroblue-tetrazolium (NBT), and L-cysteine were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). Fructose and 2,4-dinitrophenylhydrazine (DNPH) were purchased from Ajax Finechem (Taren Point, Australia). Trichloroacetic acid (TCA) was purchased from Merck (Darmstadt, Germany). OxiSelect™ CML ELISA kit was obtained from Cell Biolabs (San Diego, CA, USA). All other chemicals and solvents used in this study were of analytical grade.
Preparation of extract
Pomelo (Kao Yai) was obtained from Nakhon Phatom province and harvested at mature stage (Figure 1(Fig. 1)). The pulp of pomelo was collected by manual peeling from the peel and then homogenized using a commercial blender. The pulp was lyophilized and exhaustively extracted in a 2-step aqueous methanol process at 4 °C for 6 consecutive days. The supernatant was evaporated in a rotary evaporator at 60 °C and stored in the dark under a vacuum dessiccator at room temperature. Thereafter, the dried extract was purified to discard sugars and organic acids using a Sep-Pak C18 Cartridge (Li et al., 2006[17]).
In vitro glycation of BSA
The formation of glycated BSA was examined according to a previously described method (Adisakwattana et al., 2012[3]). Briefly, BSA (10 mg/mL) was incubated with 1.1 M fructose in 0.1 M phosphate buffered-saline, pH 7.4, containing 0.02 % sodium azide in the dark at 37 °C for 7, 14, 21, and 28 days. Before incubation, the pomelo extract was added to the mixtures. We used 10 % dimethylsulfoxide (DMSO) as a solvent for this study. The amount of glycated BSA formed was measured on the basis of the fluorescent intensity at an excitation wavelength of 355 nm and an emission wavelength of 460 nm.
Determination of fructosamine
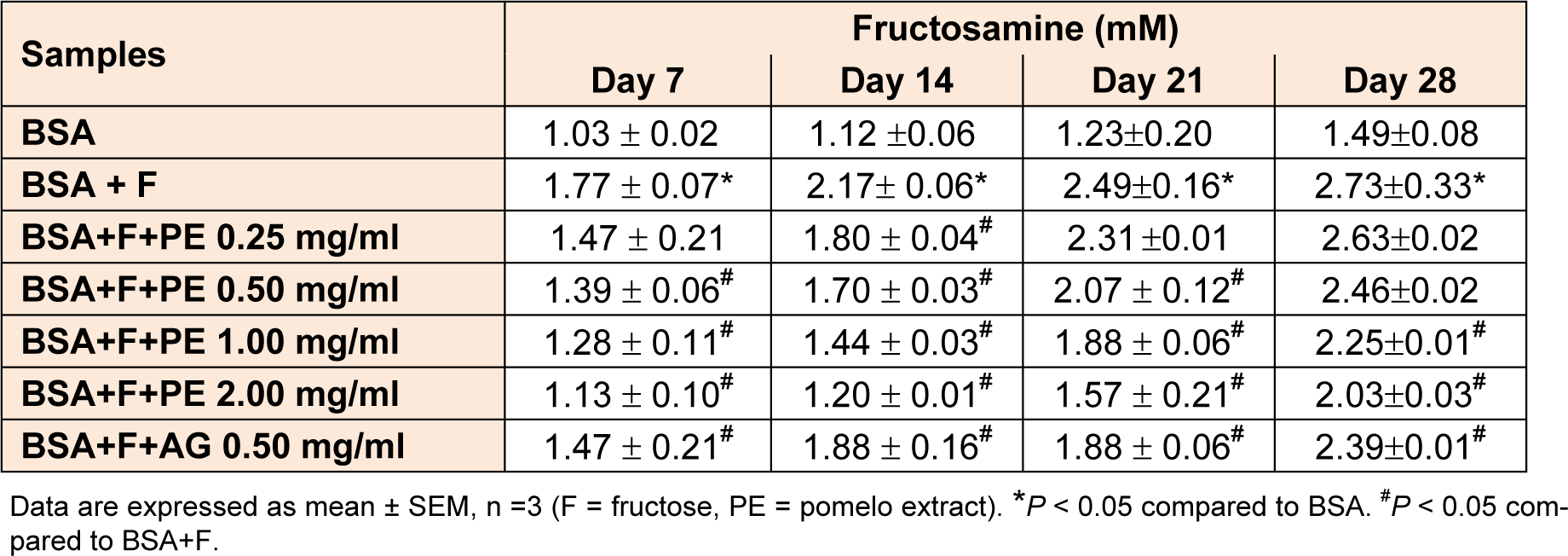
The concentration of fructosamine, the Amadori product, was measured by using the NBT assay (Adisakwattana et al., 2012[3]). Glycated BSA (10 µL) was incubated at 37 °C with 200 µL of 2.5 mM NBT in 0.1 M carbonate buffer, pH 10.4. The absorbance was recorded at 590 nm at 2 time intervals, the first reading was at 10 min and the second was at 15 min. The concentration of fructosamine was calculated using different absorption values at the 10 and 15 min time points, and these values were compared to that of 1-deoxy-1-morpholino-fructose (1-DMF) as the standard.
Determination of carbonyl content
For the assessment of the extent of protein oxidation, we measured the protein carbonyl content (Uchida et al., 1998[33]). To measure the protein carbonyl content, glycated BSA (100 L) was reacted with 400 µL of 10 mM DNPH at room temperature for 60 min. After incubation, 20 % TCA (500 µL) was added to each solution and kept in ice for 5 min. After centrifugation at 10,000 rpm for 10 min at 4 °C, the supernatant was discarded and the pellets was resuspended in 500 µL of 10 % TCA and then incubated on ice for 5 min. After incubation, the solution was centrifuged at 10,000 rpm for 10 min at 4 °C. The supernatant was discarded and the pellets were resuspended in 500 µL of ethanol/ethyl acetate (1/1) mixture and mixed thoroughly using a vortex mixer. After mixing, the solution was centrifuged at 10,000 rpm for 10 min at 4 °C. After discarding the supernatant, the process of resuspending the pellets and centrifugation was repeated twice. Then, 250 µL of 6 M guanidine hydrochloride was added to the solution and the absorbance was measured at 370 nm. The concentration of the protein carbonyl group was calculated using the extinction coefficient (ε = 22,000 M-1cm-1). The results were expressed as nmol carbonyl/mg protein.
Determination of thiol concentration
Glycated BSA (70 µL) was incubated with 2.5 mM DNTB (130 µL) in 0.1 M phate-buffered saline (PBS), pH 7.4, at room temperature for 15 min. After incubation, the absorbance was recorded at 410 nm. The concentration of free thiol was calculated by using the standard curve for l-cysteine (Sedlak and Lindsay, 1968[28]). The results were expressed as nmol/mg protein.
Determination of CML concentration
The concentration of CML, a major AGE, was determined by using an enzyme linked immunosorbent assay (ELISA) kit. The concentration of CML was calculated by using the standard curve of CML-BSA from the assay kit.
Quantification of flavonoid constituents
The flavonoid contents were determined using a slight modification of a previously described method (Zhang et al., 2011[39]). The flavonoid content in the pomelo extract was determined using high-performance liquid chromatography (HPLC) (Shimadzu, Kyoto, Japan) consisting of a binary pump (model LC-10A), an auto-injector (model SIL-10A), and a UV detector (model SPD-10A). The flavonoid separation was performed using reversed-phase Vertic SepTM UPS C-18 column (4.6 × 250 mm, 5 µm, Vertical Chromatography). The mobile phase consisted of
(A): water/acetic acid (99:1, v/v) and
(B): acetone nitrile/acetic acid (99:1, v/v).
The gradient was as follows:
0 min, 5 % B;
5 min, 8 % B;
7 min, 12 % B;
12 min, 18 % B;
17 min, 22 % B;
22 min, 25 % B;
27 min, 35 % B;
37 min, 53 % B;
38 min, 53 % B;
40 min, 55 % B;
42 min, 60 % B;
57 min, 80 % B;
60 min, 85 % B and
65 min, 85 % B.
The flow rate was 1.2 ml/min. The chromatograms were captured at 280 nm. Flavonoids were quantified by using naringin, hesperidin, neohesperidin, and naringenin as standards. Chromatographic identification and confirmation of flavonoid compounds was performed by comparing their retention times with those of authentic standards and spiking the samples with standard solutions. Eight-point calibration curves (0-250 µg/mL) were used for each standard.
Statistical analysis
Data were expressed as mean ± standard error of mean (SEM) for all experimental measurements based on triplicate results (n = 3). Significant differences were determined using one-way analysis of variance (ANOVA) followed by a post-hoc test with Tukey's range test. Significant differences were considered at P < 0.05.
Results
The effect of pomelo extract on total fluorescence intensity of glycated BSA in terms of total AGEs
The formation of AGEs was monitored weekly by measuring the fluorescence intensity. The fluorescence intensity markedly increased after incubation with BSA and fructose (Figure 2(Fig. 2)). The pomelo extract (0.25-2.00 mg/ml) significantly reduced the formation of AGEs in a concentration-dependent manner. AG (0.50 mg/mL) showed an effect similar to that of the pomelo extract (0.50 mg/mL), in that AG suppressed the formation of AGEs. The percentage inhibition of formation of AGEs by the pomelo extract (0.25-2.00 mg/mL) markedly increased in a concentration-dependent manner throughout the incubation period of 28 days (Table 1(Tab. 1)). At the same concentration of 0.50 mg/mL, the percentage inhibition by the pomelo extract was the same as that by AG at day 28.
The effect of pomelo extract on formation of Amadori products
Fructose-mediated glycation of BSA significantly increased the fructosamine level after 7 days of study (Table 2(Tab. 2)). Moreover, the fructosamine level markedly increased throughout the 28 days of experiment. The addition of pomelo extract and AG significantly suppressed the generation of fructosamine. At the end of the study period, the pomelo extract at concentration of 0.25, 0.50, 1.00, and 2.00 mg/mL reduced the level of fructosamine approximately by 3.7 %, 9.9 %, 17.5 %, and 30.0 %, respectively, whereas AG (0.50 mg/mL) decreased the level of fructosamine by 12.5 %.
The effect of pomelo extract on glycation-induced oxidative modification of BSA
The determination of carbonyl content and thiol groups was used to assess the protein oxidation that occurred during the process of glycation. The carbonyl content of glycated BSA significantly increased compared to that with BSA alone at day 28 (Table 3(Tab. 3)). Furthermore, the pomelo extract significantly decreased the protein carbonyl content at concentration of 1 and 2 mg/mL. The percentage reduction of carbonyl content by pomelo extract at concentration of 1.00 and 2.00 mg/mL was 9.2 % and 28.5 %, respectively, whereas that by AG (0.50 mg/mL) was 11.1 %. The effects of pomelo extract on the oxidation of protein thiols are shown in Table 3(Tab. 3). At day 28, compared to BSA alone, BSA incubated with fructose significantly decreased the level of thiol group. A significant increase (20.0 %) was observed in the level of thiol group in fructose-induced glycated BSA plus pomelo extract (2.00 mg/mL).
The effect of pomelo extract on CML formation
CML has been used as a biomarker for the formation of the non-fluorescent AGEs on 28 day. The pomelo extract at a concentration of 0.50 mg/mL significantly inhibited the formation of CML by 90.0 % in BSA/fructose (Figure 3(Fig. 3)). In addition, AG (0.50 mg/mL) decreased the level of CML by approximately 61.0 %.
HPLC chromatogram
The flavonoid composition of pomelo extract was analyzed using HPLC (Figure 4(Fig. 4)). Comparison of the RT and UV spectra of compound 1, 2, 3, and 5 with those of the standard indicated that these compounds were naringin, hesperidin, neohesperidin, and naringenin, respectively. The results revealed that neohesperidin (25.40 ± 0.12 mg/g dried extract) was a major compound in the pomelo extract followed by naringin (11.90 ± 0.21 mg/g dried extract), hesperidin (12.04 ± 0.12 mg/g dried extract), and naringenin (9.20 ± 0.19 mg/g dried extract).
Discussion
In this study, we examined the antiglycation effect of pomelo extract against fructose-mediated non-enzymatic glycation and oxidation-dependent damage to BSA. We determined the effects of pomelo extract during the early stage (Amadori products), middle stage (protein oxidation), and last stage (the cross-linking compound) of AGE formation (Brownlee, 1995[8]; Wu et al., 2011[34]). Reducing sugars are important modulators of non-enzymatic glycation that eventually results in irreversible formation of AGEs. Fructose, like other reducing sugars, can react with proteins through non-enzymatic glycation, which may account for accelerating aging and chronic diabetic complications (Suarez et al., 1989[30]; Yeboah et al., 1999[38]). Fructose spontaneously reacts with the amino group of BSA forming Schiff bases that spontaneously rearrange to Amadori products, also called fructosamine. Fructosamine is clinically used as an indicator for short-term control of blood glucose level in diabetic patients (Ardestani and Yazdanparast, 2007[5]). Reduction in fructosamine levels is a therapeutic strategy to delay the progression of vascular complications (Shield et al., 1994[29]). Furthermore, the formation and accumulation of AGEs is enhanced by chronic hyperglycemia (Fu et al., 1994[12]). CML is one of the most abundant AGEs that is formed between a reactive carbonyl intermediate and an amino acid residue during the formation of a Schiff base (Reddy et al., 1995[26]). In addition, many studies have shown that CML is closely related to the pathogenesis of the diabetes complications, retinopathy, and nephropathy (Monnier et al., 2005[21]).
Besides the formation of AGEs, reactive carbonyl intermediaries and protein carbonyl derivatives also cause protein modifications that are particularly prone to oxidative reactions with an amino acid such as cysteine. This amino acid is an important component of protein that maintains the protein structure, enzymatic activity, and antioxidant activity. However, it can be destroyed by the oxidation, which results in the release of free thiol groups (Dalle-Donne et al., 2003[10]). The oxidation of thiol groups results in conformational changes in protein structure, which create disulfide bonds, and generate high levels of free radicals (Murphy and Kehrer, 1989[22]). Thus, the increased levels of oxidized thiol groups are reflective of high oxidative stress, protein oxidative damage, and formation of AGEs. Therefore, major molecular modifications in the protein structure can be determined by measuring the level of protein carbonyl groups and the loss of protein thiol groups, which is the direct reflection of the high amounts of free radical generation.
Pomelo, a native citrus fruit to Southeast Asia and one of the largest Citrus species, is widely cultivated and utilized in Thailand. In addition, citrus flavonoids have been identified in the pulp of pomelo, such as naringin, narirutin, neohesperidin, and kaempferol (Abeysinghe et al., 2007[1]). The flavonoids identified in our study are consistent with those reported previously (Kim et al., 2009[16]; Zhang et al., 2011[39]). A comprehensive previous study related to naturally occurring polyphenols and flavonoids showed that these agents could reduce the formation of fructosamine, which may be related with their antiglycoxidative properties (Wu et al., 2011[34]). Previous studies indicate that natural flavonoids may play a role in the prevention of diabetic complications because flavonoids are capable of affecting multiple mechanisms in the inhibition of glycation. Sugar-mediated non-enzymatic protein glycation and oxidation can be inhibited effectively when flavonoids are added during the glycation process (Wu and Yen, 2005[35]). Matsuda et al. (2003[20]) suggest that the structural requirements of flavonoids for inhibition of protein non-enzymatic glycation include hydroxyl groups at the 3′-, 4′-, 5-, and 7-positions, which increases the inhibitory activities. In addition to the structure-activity relationships, flavones are more stronger than flavonols, flavanones, and isoflavones (Matsuda et al., 2003[20]). These findings are in agreement with another study, which shows that flavonoids suppress the total fluorescence AGEs in the order flavone > flavonol > flavanol > flavanone (Wu and Yen, 2005[35]). A recent study in type 1 diabetes patients showed that daflon 500, which is made up of the flavonoids diosmin (90 %) and hesperidin (10 %), decreases the level of hemoglobin A1c (HbA1C) (an Amadori product) accompanied by an increase in glutathione peroxidase levels (Manuel y Keenoy et al.,1999[19]). Our findings indicate that pomelo extract reduces the level of fructosamine and the formation of CML and decreases the formation of AGEs. Accordingly, the inhibitory activity of pomelo on the formation of AGEs and protein oxidation might be associated with its flavonoid content
A number of recent studies indicate that trapping free radicals and inhibiting the formation of reactive carbonyl groups using antioxidant compound is one of the strategies for inhibition of protein glycation. Previous studies have documented the antioxidant activity of pomelo through its ability to inhibit the formation of free radicals generated by 1,1-diphenyl-2-picrylhydrazyl radical and to inhibit the oxidation of 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS+●) (Jayaprakasha et al., 2008[13]; Lim et al., 2006[18]). Moreover, the oxygen radical absorbance capacity (ORAC) assay showed that the pomelo extract decreases peroxyl radicals (Jayaprakasha et al., 2008[13]). The pomelo extract exhibits a high ability to reduce Fe3+ to Fe2+, which suggests its ability to suppress the progression of Fenton reaction and thus impeding the formation of a highly reactive hydroxyl radical (Pichaiyongvongdee and Haruenkit, 2009[ref:25]). Flavonoids in the pomelo extract have shown antioxidant activity, which is directly related to their ability to inhibit free radical formation and lipid peroxidation. For example, hesperidin and neohesperidin dihydrochalcone effectively inhibit formation of superoxide and oxygen species (Suarez et al., 1998[31]). In fact, flavonoids can trap reactive oxygen species and free radicals in various ways. One way is the direct scavenging of free radicals because of the phenolic hydroxyl groups in their structure, which readily donate electrons to the unstable free radical and thus the free radical becomes more stable and less-reactive (Nijveldt et al., 2001[23]). Previous studies, which support the proposed mechanisms of antiglycation by flavonoids mainly attribute this effect to the potent antioxidant activities of the flavonoids (Wu and Yen, 2005[35]). The scientists have revealed that flavonoids show strong abilities to scavenge free radicals formed during glycation and prevent the self-oxidation of reducing sugars (Peng et al., 2011[24]). According to these evidences, our findings suggest that the inhibitory effects of flavonoids in the pomelo extract on the AGE formation and protein oxidation are remarkably related to their scavenging abilities of free radicals formed during the glycation process. However, the antioxidant activity of flavonoids in the pomelo extract might not be the only reason for the antiglycation effect. The recent mechanisms underlying antiglycation include breaking the cross-linking structures in the formed AGEs, blocking the carbonyl or dicarbonyl groups in reducing sugars, Schiff bases, and inhibiting the formation of late-stage Amadori products (Peng et al., 2011[24]; Wu et al., 2011[34]). Further comprehensive studies of flavonoids are required to evaluate the antiglycation mechanisms described above.
Conclusions
Our study describes the antiglycation effect of pomelo extract in the BSA/fructose system. Our findings showed marked inhibition of the formation of AGEs, fructosamine, and CML. The pomelo extract decreased the protein carbonyl content and prevented the loss of protein thiol in BSA. The pomelo extract has potential to be an antiglycation agent and offers remarkable prospects for prevention of pathogenesis in conditions associated with diabetic complications and aging.
Acknowledgements
We wish to thank the Thailand Research Fund (TRF) for financial support (RDG5420029). The authors would also like to thank The Medical Food Research and Development Center, and The Research Group of Herbal Medicine for Prevention and Therapeutic of Metabolic diseases, which have been financially and institutionally supported by Chulalongkorn University.
References
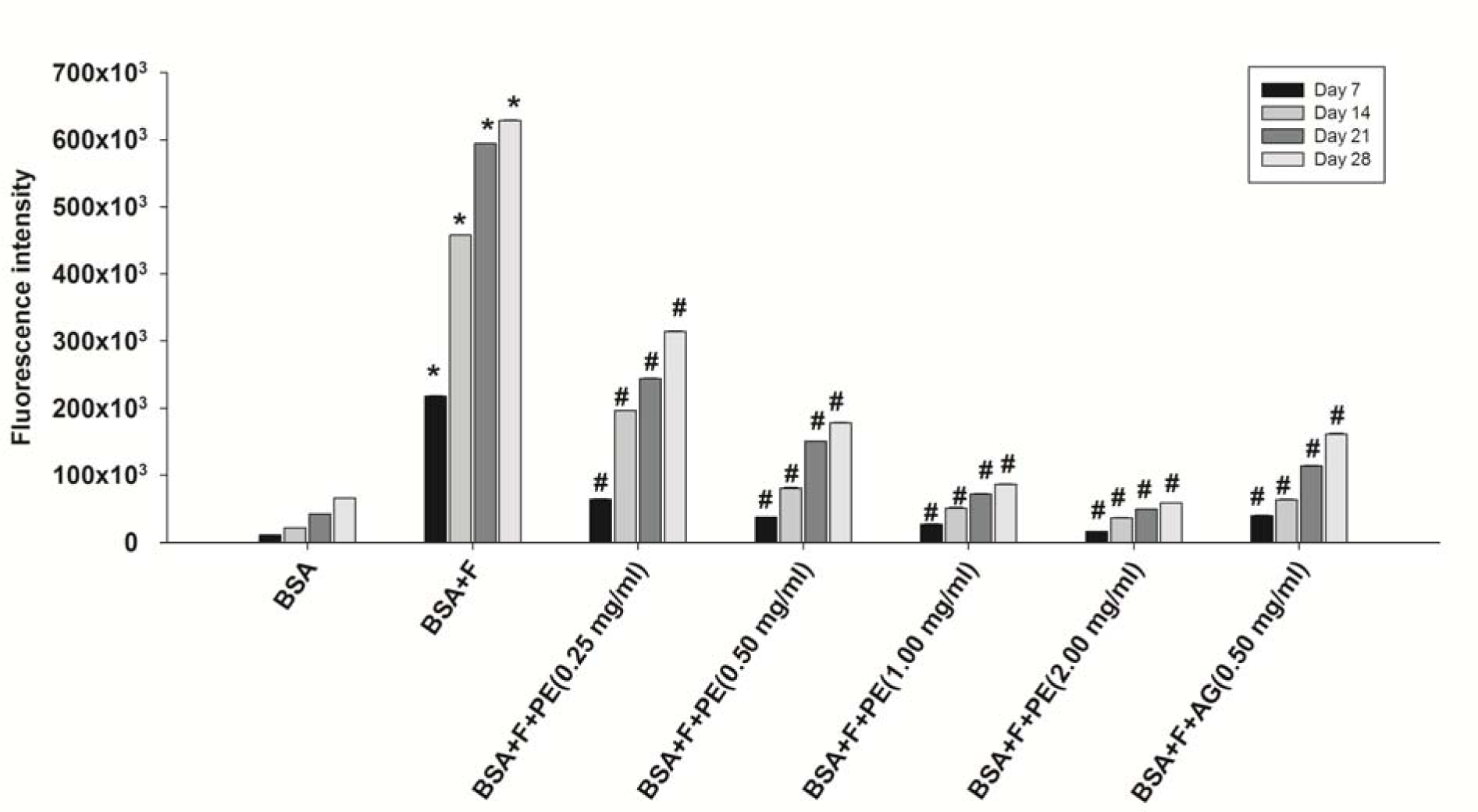
Figure 2: Effects of pomelo extract on fluorescent modification derived from formation of advanced glycation end products (AGEs) in bovine serum albumin (BSA) incubated with fructose. Each value represents the meastandard errorn ± (SE, n = 3). *P < 0.05 compared to BSA alone at the same week, # P < 0.05 compared to BSA+F (Fructose) at the same week
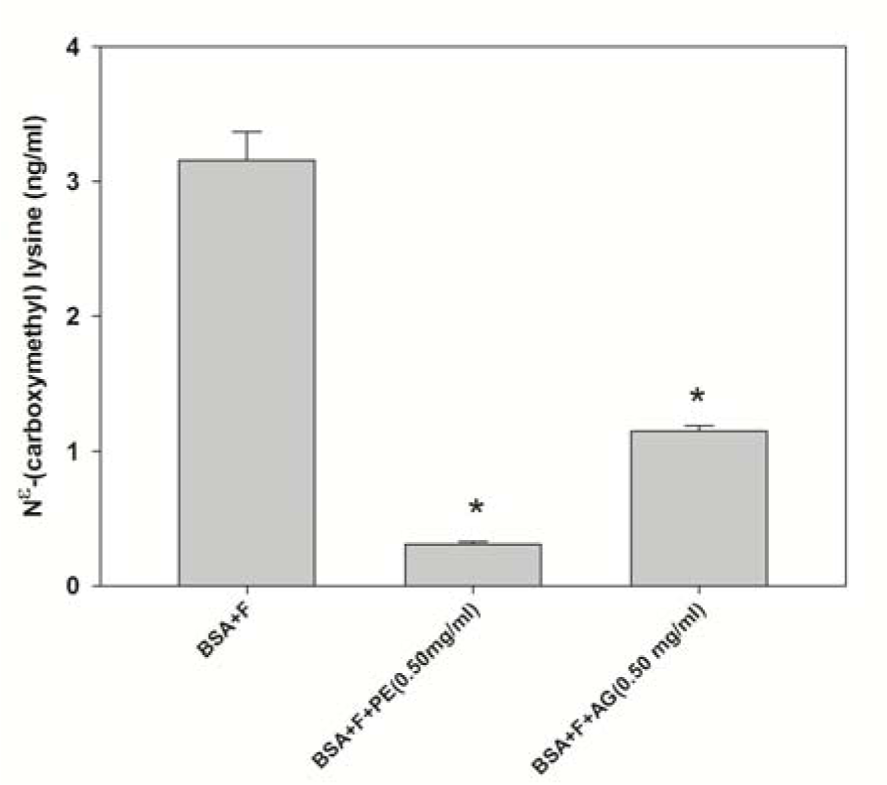
Figure 3: Effects of pomelo extract on the level of Nε-(carboxymethyl) lysine (CML) in bovine serum albumin (BSA) incubated with fructose after 28 days. Each value represents the mean ± standard error of mean (SEM, n = 3). *P < 0.05 compared to BSA+F (Fructose)
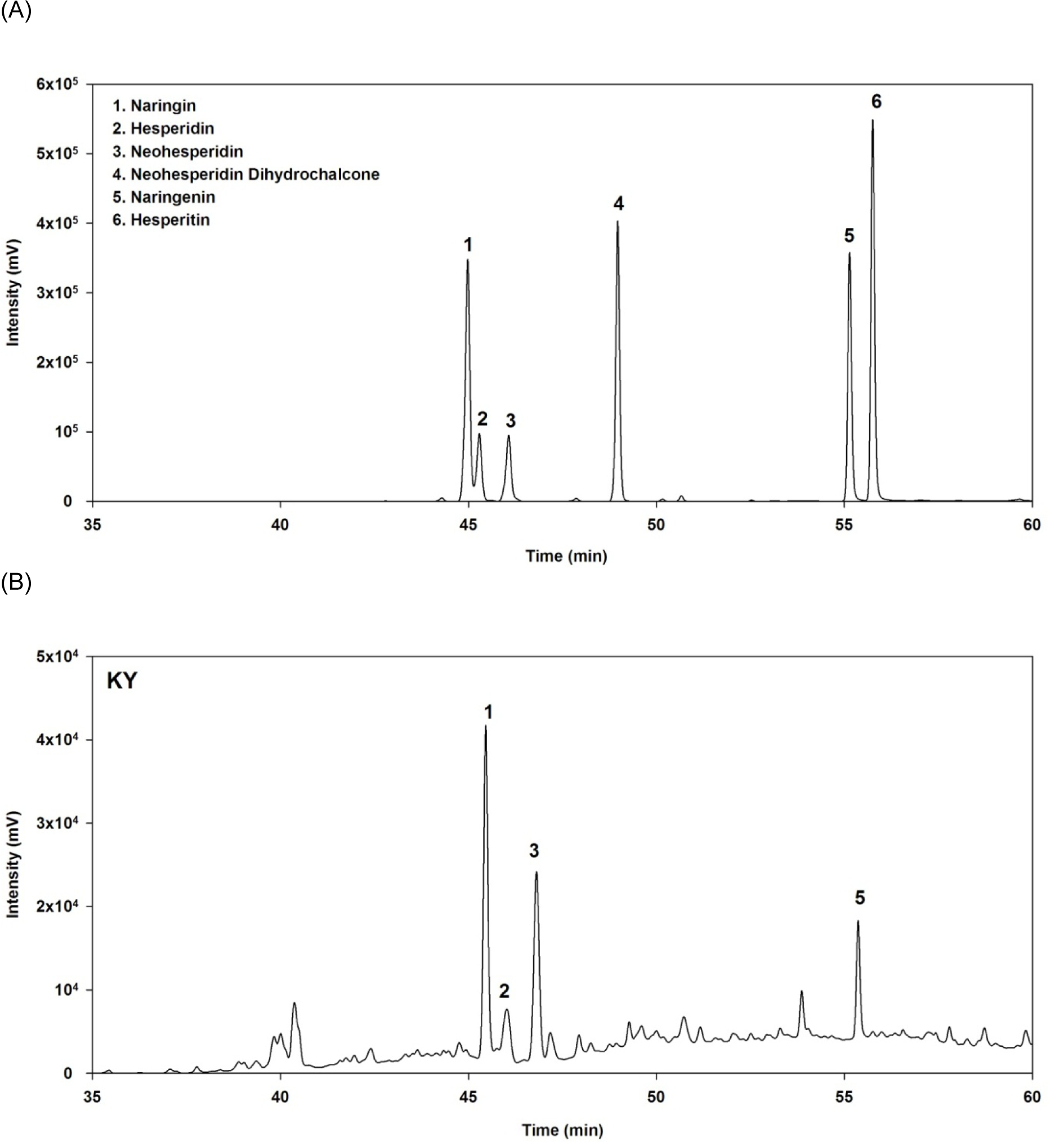
Figure 4: (A) High-performance liquid chromatography (HPLC) chromatograms at 280 nm of standard compounds at a concentration of 250 µg/mL and (B) the pomelo extract (2 mg/mL).
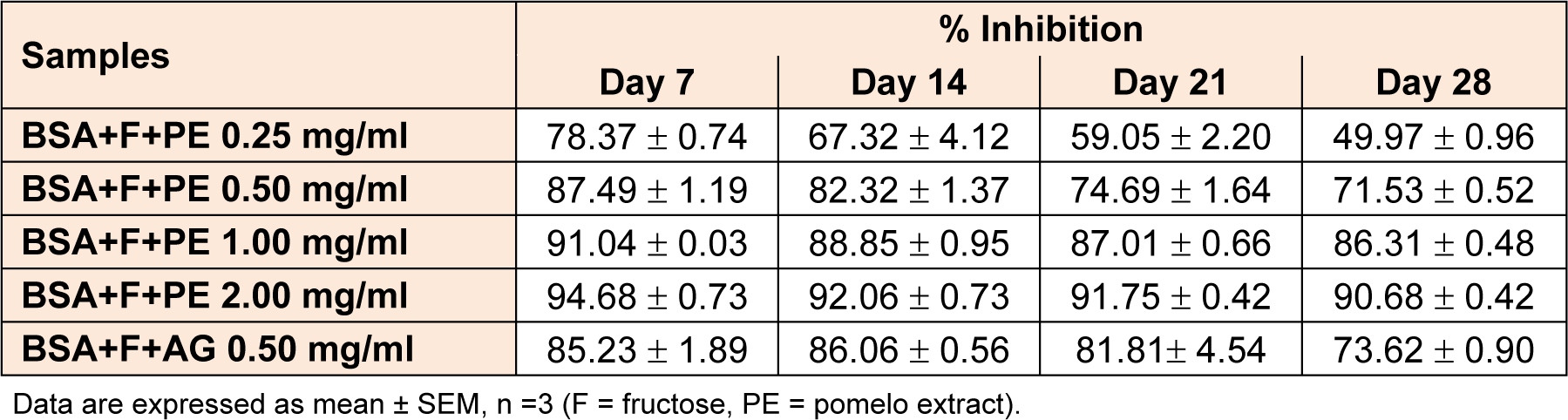
Table 1: The percentage inhibition of pomelo extract on the formation of fluorescence AGE at day 7, 14, 21, and 28
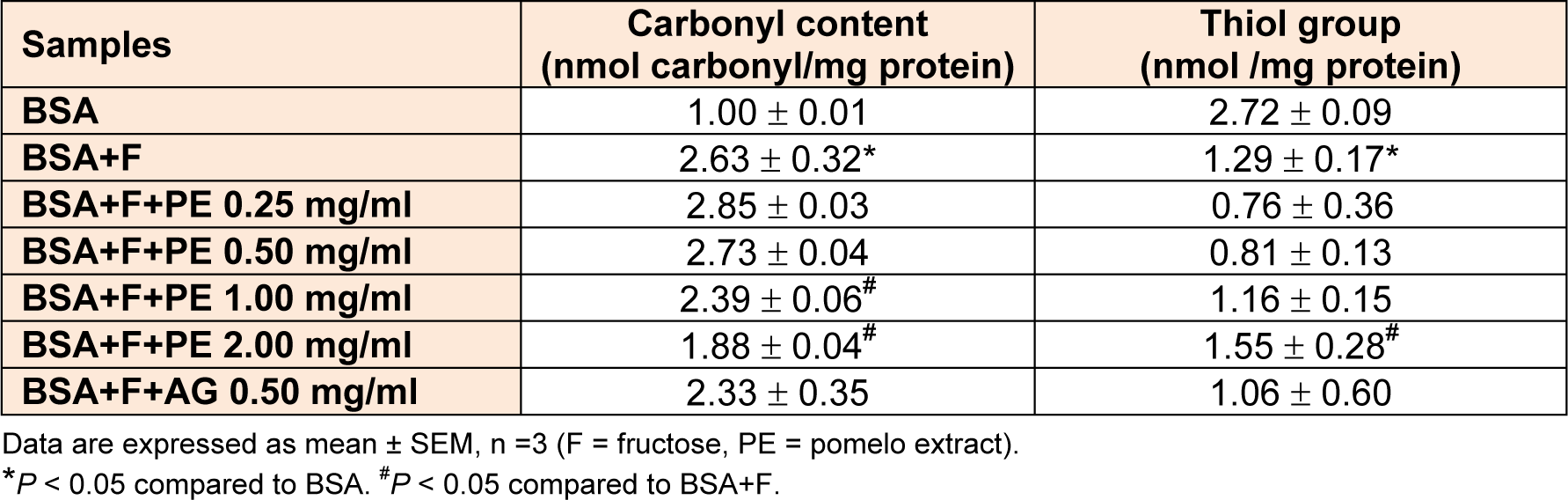
Table 3: The effect of pomelo extract on the formation of carbonyl content and the level of thiol group at day 28
[*] Corresponding Author:
Sirichai Adisakwattana, Research Group of Herbal Medicine for Prevention and Therapeutic of Metabolic Diseases, Chulalongkorn University, Bangkok, 10330, Thailand; Tel.: +66-2-218-1067; Fax: +66-2-218-1076, eMail: Sirichai.a@chula.ac.th