Case report
Hepatotoxicity induced by zoledronic acid in an aged woman with primary osteoporosis
Yanhui Lu1, Yu Pei1, Yinghong Shao1, Shuangtong Yan1, Lichao Ma1, Fusheng Fang1, Mengmeng Jin1, Minyan Liu1, Jian Li1, Chunlin Li1[*]
1Department of Geriatric Endocrinology, Chinese PLA General Hospital, No. 28 Fuxing Road, 100853 Beijing, ChinaEXCLI J 2013;12:Doc115
Abstract
Zoledronic acid, a bisphosphonate, has been approved for treatment and prevention of osteoporosis. This case describes a 73-year-old woman with primary osteoporosis who developed transient hepatotoxicity after zoledronic acid (ZOL) treatment. Three days after ZOL infusion, aspartate aminotransferase (AST), alanine aminotransferase (ALT), and gamma-glutamyltransferase (GGT) were increased by 9.9, 8.1, and 3.7 times, respectively, compared with pretreatment values. Liver protective agents were administered. The aminotransferase returned within normal ranges 12 days post-infusion. Currently, the relationship of ZOL and liver damage is not quite clear, which cannot be explained by its pharmacokinetics. The aim of this case report is to increase the clinician's awareness of the possible adverse effect on the liver, and ZOL should be cautiously administered in patients with liver disease.
Keywords: bisphosphonates, osteoporosis, hepatotoxicity
Introduction
Bisphosphonates are widely used for the treatment of osteoporosis, Paget's disease, metastatic bone disease, multiple myeloma and hypercalcaemia of malignancy. Intravenous zoledronic acid (5 mg once yearly) have also been approved for the treatment of osteoporosis. The overall safety and tolerability of bisphosphonate treatment for osteoporosis has been very good and any serious adverse events related to this therapy are rare. Until now, a few cases of hepatitis developing several months (Halabe et al, 2000[4]; Carrére et al., 2003[1]; Yanik et al., 2007[7]) or years (Phillips, 2007[5]) after starting bisphosphonate therapy and resolving several months after discontinuing bisphosphonates have been reported. Liver biopsy (Halabe et al, 2000[4]; Yanik et al., 2007[7]) revealed lesions suggestive of a drug effect.
Here we report the case of an aged female patient with primary osteoporosis who developed transient hepatotoxicity after zoledronic acid (ZOL) treatment.
Case report
The primary osteoporosis diagnosis was established in a 73-year-old Chinese postmenopausal woman in December 2011. Her bone mineral density: The T score of the femoral head was -2.6. The patient had no severe back pain or fractures. She had no history of any obvious predisposing factors or liver diseases. She had no history of smoking and drinking alcohol. And she had no family history of osteoporosis. Physical examination was normal.
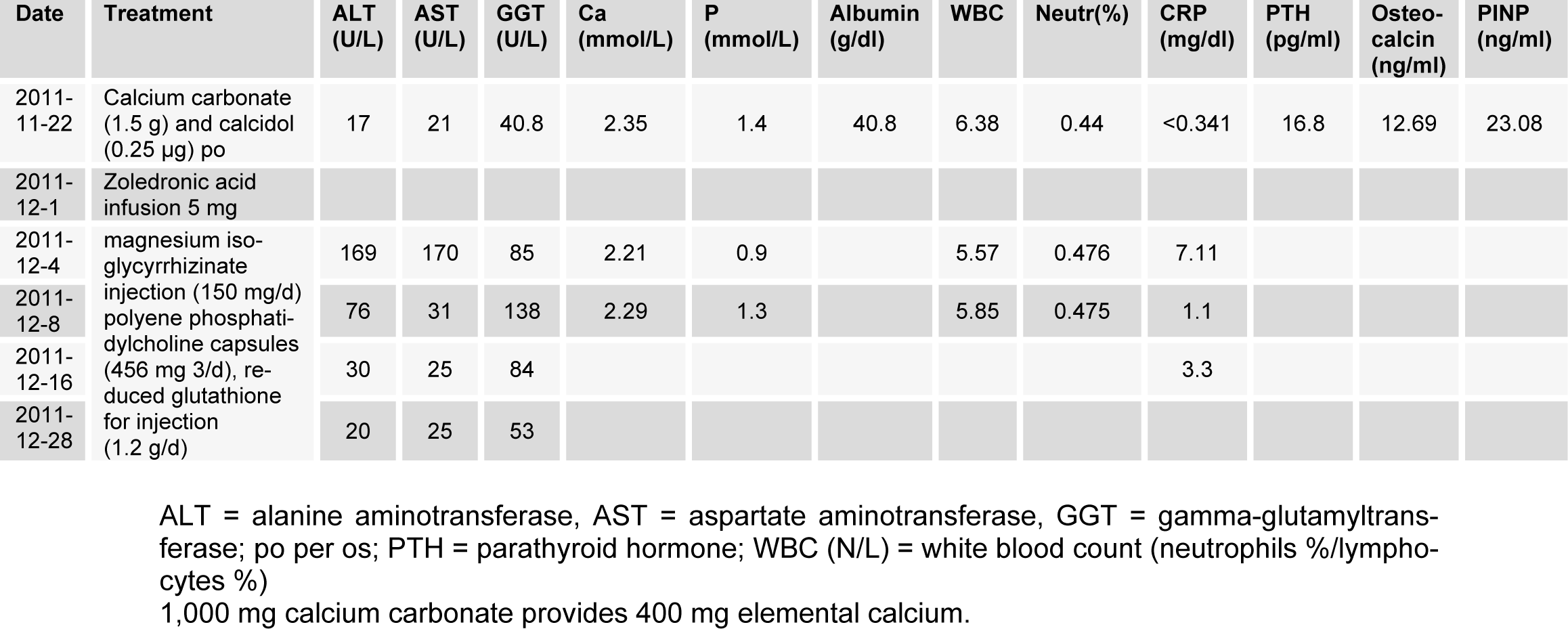
The patient was administered per os calcium and cholecalciferol supplements 2 months before ZOL infusion. The patient was not treated with aceraminophen or other nonsteroidal anti-inflammatory drugs prior to or after ZOL infusion. At the day after infusion, she experienced a transient flu-like syndrome, self-limited less than 3 days. Three days after ZOL infusion, C-reactive protein (CRP) increased to 7.11 mg/dl (normal range 0-0.8 mg/dl), while aspartate aminotransferase (AST), alanine aminotransferase (ALT), and gamma-glutamyltransferase (GGT) were increased by 9.9, 8.1, and 3.7 times, respectively, compared with pretreatment values (Table 1(Tab. 1)). The abdominal US revealed only hepatic mild homogenous brightness without focal lesion of the liver or biliary ducts. Serologic tests for hepatitis B and C viruses were normal. Liver protective agents (magnesium isoglycyrrhizinate injection (150 mg/d)), polyene phosphatidylcholine capsules (456 mg 3/d), reduced glutathione for injection (1.2 g/d) were administered. The CRP, AST, and ALT returned within normal ranges 12 days post-infusion, and the GGT returned within normal range 24 days post-infusion (Table 1(Tab. 1)).
Discussion
Intravenous bisphosphonates, used in oncology and for the treatment of osteoporosis, have been associated with adverse events such as acute phase response, hypocalcaemia and secondary hyperparathyroidism, musculoskeletal pain, osteonecrosis of the jaw and ocular events. Moreover, zoledronic acid has been associated with renal toxicity. The flu-like symptoms observed following initial exposure to zoledronic acid are associated with elevations in serum TNF-alpha and IL-6 (Dicuonzo et al., 2003[2]). Our data support an inflammatory element to this phenomenon in aged female, with an elevation in CRP. Up to now, there are only a few cases of transient hepatotoxicity following ZOL infusion in human. In this case, indeed, the patient remained asymptomatic, while the CRP, ALT, AST, and GGT were transiently increased. ALT, AST, and GGT increased 3 days after ZOL, and other potential causes of hepatotoxicity could be excluded, which indicates ZOL as a cause of liver injury.
Biopsy-confirmed hepatotoxicity after ZOL was previously shown in rats (Dieterle et al., 2007[3]). Polyzos et al. (2011[6]) reported that a patient with Paget's disease of bone developed transient hepatotoxicity after zoledronic acid (ZOL) treatment, her AST, ALT, and GGT were increased by 8.1, 6.7, and 6.7 times, respectively one day after infusion, and were normalized 7 days post-treatment. There were no serious adverse events due to liver disease reported, and the clinical significance of such slight increase is probably minor.
The bisphosphonates are essential in the treatment of postmenopausal osteoporosis. The positive impact of bisphosphonates on the management of millions of patients with osteoporosis and other metabolic bone disorders has been enormous. Currently, the relationship of ZOL and liver damage is not quite clear, which cannot be explained by its pharmacokinetics. Idiosyncratic sensitivity to ZOL or adjuvant excipients were suspected. The aim of this case report is to increase the clinician's awareness of the possible adverse effect on the liver, and ZOL should be cautiously administered in patients with liver disease.
Conflict of interest
The authors declare no conflict of interest.
References
[*] Corresponding Author:
Chunlin Li, Department of Geriatric Endocrinology, Chinese PLA General Hospital, No. 28 Fuxing Road, 100853 Beijing, China; Tel.: +86 0101381-0921-655, eMail: lcl301@yahoo.com.cn