Research article
Enzymatic saccharification and lactic acid production from banana pseudo-stem through optimized pretreatment at lowest catalyst concentration
Muhammad Idrees1[*], Ahmad Adnan1, Farnaz Malik2, Fahim Ashraf Qureshi3
1Department of Chemistry, GC University, Katchery Road, Lahore 54000, Pakistan2Drug Control and Traditional Medicine Division, National Institute of Health, Chak Shahzad, Islamabad, Pakistan
3Office of Research, Innovation and Commercialization, COMSATS Institute of Information Technology, Chak Shahzad, Park Road, Islamabad 45600, Pakistan
EXCLI J 2013;12:Doc269
Abstract
This work estimates the potential of banana pseudo-stem with high cellulosic content 42.2-63 %, for the production of fermentable sugars for lactic acid production through statistically optimized pretreatment method. To evaluate the catalyzed pretreatment efficiency of banana pseudo stem based on the enzymatic digestibility, Response Surface Methodology (RSM) was employed for the optimization of pretreatment temperature and time using lowest concentrations of H2SO4, NaOH, NaOH catalyzed Na2S and Na2SO3 that seemed to be significant variables with P<0.05. High F and R2 values and low p-value for hydrolysis yield indicated the model predictability. The optimized condition for NaOH was determined to be conc. 1 %, temperature 130 oC for 2.6 hr; Na2S; conc. 1 %, temperature 130 oC for 2.29 hr; Na2SO3; conc. 1 %, temperature 130 oC for 2.41 hr and H2SO4; conc. 1 %, temperature 129.45 oC for 2.18 hr, produced 84.91 %, 85.23 %, 81.2 % and 76.02 % hydrolysis yield, respectively. Sulphuric acid provided 33+1 gL-1 reducing sugars in pretreatment step along with 38+0.5 gL-1 during enzymatic hydrolysis. Separate hydrolysis and fermentation of resulting sugars showed that the conversion of glucans into lactic acid reached 92 % of the theoretical yield of glucose.
Keywords: fermentable sugars, optimized pretreatment, digestibility, lactic acid, glucans
Introduction
Agricultural residue is gaining much importance these days because of low cost, nonfood nature, easy and decentralized availability for the biological production of industrial chemicals such as lactic acid, acetic acid, propionic acid and ethanol fuel (Wyman et al., 1992[38]). The digestibility of cellulose present in lignocellulosic biomass is hindered by many physicochemical, structural, and compositional factors. In the conversion of lignocellulose into fermentable sugars, the biomass needs to be pretreated so that the cellulose in the plant fibers is exposed for further treatments. The pretreatment uses a number of techniques including biological treatment, and steam explosion to alter the structure of cellulosic biomass to make cellulose more accessible, such that enzymes digest the cellulosic biomass completely for production of sugars (Parveen et al., 2009[28]).
Enzymatic digestibility has been enhanced for wheat straw, corn stover, bagasse, sunflower stalks, switchgrass, coastal Bermuda grass, cotton stalks, and hardwoods using sodium hydroxide pretreatment (Teater et al., 2011[33]). Thus, dilute alkali pretreatment has been widely accepted as one of the better ways to pretreat the lignocellusic materials for fuel (Sun and Cheng, 2002[31]). While some prefer to pretreat the biomass with dilute acids at temperature ranging 100-200 °C to disrupt the lignin-carbohydrate network for maximum hydrolysis of cellulose into fermentable sugars (Kootstra et al., 2009[17]).
Banana pseudo stem contains average amount of cellulose 47 %, hemicellulose 13 %, holocellulose 55 %, lignin 13.0 %, ash 8.2 % and extractives 3.05 % (Bernstad et al., 2012[5]). This is an abundant and cheap resource of lignocellulosic biomass that can be found throughout the world especially in Asia and Europe. In Pakistan, the planting area for banana stem was 33,000 acres (Jatoi, 2008[15]) while in India it was the second largest fruit crop. Due to its high cellulosic contents a large number of applications is under study such as manure (Ultra et al., 2005[36]), feed (Ulloa et al., 2004[35]), kraft pulping (Guha, 1960[11]), chlorine free formic acid pulping (Jahan et al., 2007[14]), synthesis of sodium carboxymethylcellulose (CMC) (Adinugraha et al., 2005[2]). Pseudo stem, present in the crop fields create environmental pollution, producing hydrogen sulfide and ammonia gas after decaying (Li et al., 2010[20]; Tock et al., 2010[34]; Baig et al., 2004[4]; Ilori et al., 2007[13]). Nevertheless, studies on the sugar production from pseudo stem are few. Present study deals with the pretreatment of banana stems. Enzymatic hydrolysis of the treated material was investigated to determine its potential for lactic acid production.
This study focused on acid, alkali, alkali catalyzed sulphide and sulphite pretreatment in order to establish the most favorable pretreatment condition by using the Response Surface Methodology. The objectives of this study were to:
1. find the suitable catalyst for pretreatment of Banana pseudo stem,
2. statistically determine the optimized pretreatment conditions; lowest catalyst concentrations (%), pretreatment time, and optimal temperature, and
3. lactic acid production from resulting sugar.
Material and Methods
Chemicals
All chemicals used were of analytical grade and used without further purification. Concentrated sulphuric acid (95-97 wt %) [CAS 7664-93-9], D(+)-Glucose [141341] from Panreac and α-naphthol were purchased from Merck GmbH (Darmstadt, Germany). 3,5-Dinitrosalicylic acid [609-99-4] and analytical grade Phenol [CAS 108-95-2] were procured from Fluka Chemie GmbH), D(+)-Xylose (GPR) from BDH Ltd (England) and L-(+)-Arabinose was obtained from Sigma Aldrich [CAS 5328-37-0]. Two types of enzymes, ACCELLERASE 1500 and OPTIMASH™ BG were obtained from Genencor International Inc. whereas; Lactobacillus acidophilus ATCC 4356 was obtained from Microbiologies, Inc USA (Lot#256053).
Banana pseudo stem
Banana stem was collected from a local farm from Nankana Sahib District, Punjab, Pakistan. After collection, the banana stem was dried in sunlight for five days, cut into small pieces and ground by using pulveriser equipped with 150 mesh steel sieves. The dried powdered material was then stored at room temperature away from sunlight.
Optimization of pretreatment process
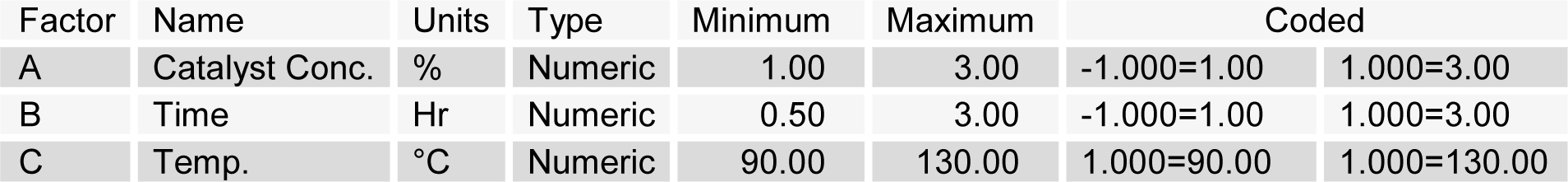
The catalyst (H2SO4, NaOH, NaOH 0.5 % catalyzed Na2S and Na2SO3) concentration (X1), time (X2) and temperature (X3) used for pretreatment was optimized by using central composite design experiment to enhance the enzymatic hydrolysis yield. The design matrix with eighteen experimental runs in two blocks with four replicates of the midpoint was used. Twenty five grams of Banana stem powder was mixed with predefined catalyst solution in 1:10 (w/v) ratio, in 500 ml flask separately. The flasks were autoclaved (CL-40L (ALP Co, Ltd. Tokyo Japan) at different temperatures (90-130 oC at pressure 0.13-0.2 MPa) for different time intervals. The solution in flasks was cooled and filtered with Whatman filter paper. The residue was washed with distilled water for several times to bring the pH at 4.8. The residue was dried at 105 °C for 20 min and weighed. Coded values of independent variable along with their minimum and maximum values are shown in Table 1(Tab. 1). The model, used to enhance the response by optimizing the pretreatment factors was a second-order polynomial as follows;
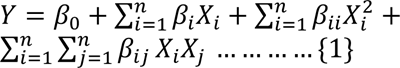
where Y is the Hydrolysis yield and i,j are linear and quadratic coefficients respectively, β0 is the regression coefficient, X1-X3 are the coded factors under study. Regression analysis and estimation of the coefficient were performed using Design Expert Software 8.1.07.
Enzymatic hydrolysis of pretreated material
Accellrase 1500 has multiple enzyme activities and is effective for cellulose, hemicellulose and β-glucans. OPTIMASH™ BG is a mixture of Xylanase and ß-Glucanase which was used together with ACCELLERASE 1500 to enhance enzymatic hydrolysis efficiency of hemicellulose. The enzymatic hydrolysis was carried out in 250 mL Erlenmeyer flask. Accellrase 1500, 0.2 mLg-1 dry weight of biomass and 0.1 mLg-1 Optimash BG were used for hydrolysis. The pH of the reaction mixture was set at 4.8 by adding 100 mL of 0.1 M acetate buffer solution after adding five grams of treated dry mass. The flasks were kept in orbital shaker for 48 hours at 50 °C with 160 rpm. After regular time intervals, samples were taken from each flask and kept in boiling water to inactivate the enzyme. Each sample was filtered on a Whatman filter paper 1 and subsequently analyzed. Each experiment was performed in triplicates.
Lactic acid fermentation
Lactobacillus acidophilus, a homofermentative, lactic acid producing bacteria (Lee et al., 2001[19]) was used for the production of lactic acid from enzymatic hydrolyzate. Inoculum was prepared by transferring cells into 100 mL flask containing 50 mL of culture medium containing 10 gL-1 yeast extract, 2 gL-1 (NH4)2HPO4, 0.1 gL-1 MnSO4 and 30 gL-1 glucose and was subsequently incubated at 37 oC for 12 hours (Wee et al., 2006[37]). After two consecutive transfers to fresh medium, sample from mixture was used to inoculate the fermentation medium. Cellulosic hydrolyzate, obtained from enzymatic hydrolysis of each pretreated sample, supplemented with 10 gL-1 yeast extract, 2 gL-1 (NH4)2HPO4, 0.1 gL-1 MnSO4 was used for fermentation. The inoculum to solution ratio of 1:20 was used for fermentation purposes. Samples, for glucose and lactic acid analysis, were taken at specific intervals during 72 hours fermentation process.
Analysis of monomeric sugars and lactic acid
Chemical analysis (components; lignin, hemicellulose and cellulose) of the biomass was determined through Lin et al., (2010[21]) method. Reducing sugars were determined by using Ghose (1987[9]) method. 3,5-dinitrosalicylic acid (DNS) was used as a coloring reagent, the absorbance for each sample was recorded at λ=546 nm with double beam spectrophotometer (Cecil Aquarius CE 7200). Identification of monosaccharide contents was determined with the help of thin layer chromatography (TLC) in pretreated, enzymatic hydrolyzate and fermentation media. The mobile phase for TLC analysis consisted of acetonitrile solution (acetonitrile: water, 85:15 v/v), using a 20×20 cm Kieselgel 60F 254 (Merck) as a TLC plate. The plates were soaked in 0.5 % α-naphthol and 5 % H2SO4 in ethanol and then dried in oven at 80 °C for 5 min (Idrees et al., 2013[12]). Sugar yield was calculated on pretreated solid biomass used for enzymatic hydrolysis by using the following equation (Dedsuksophon et al., 2011[6]).
Sugar Yield ( %) = 100 (sugar produced during hydrolysis/gram of biomass feedstock)
Lactic acid was quantified with the help of HPLC (LC- 20AT, Shimadzu) method used by Bai et al. (2000[3]). Reverse phase SMA-C18 column with size 4.6×250 mm of SMT, coupled with a UV variable wavelength detector (SPD-M20A, Shimadzu) at 210 nm was employed. Elution was carried out with the help of 0.01 M phosphoric acid having pH 2.5 with flow rate of 1ml/min at room temperature.
Results and Discussion
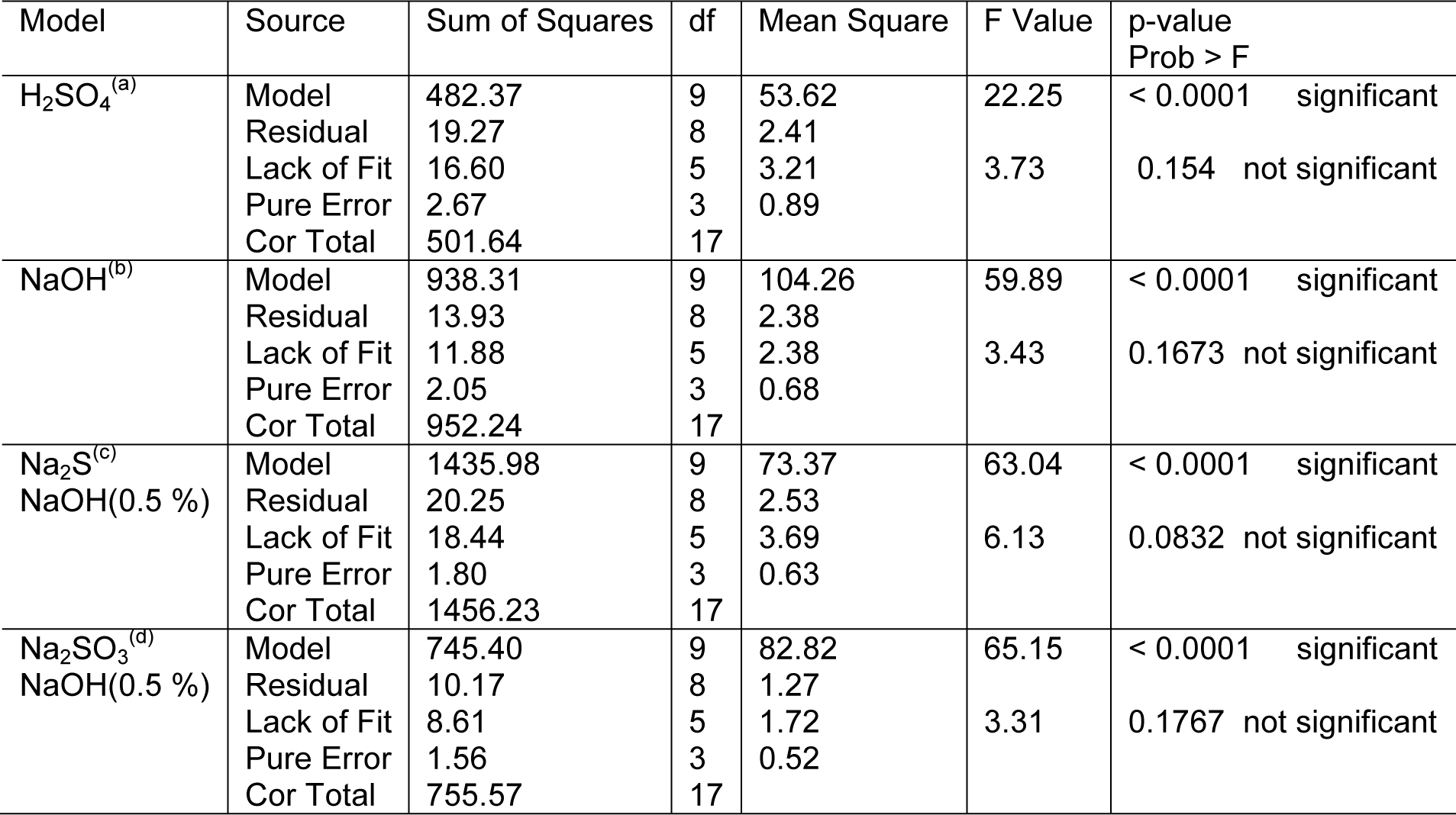
The high cellulosic content of (46-72 %) (Li et al., 2010[20]) banana stem was considered as a potential source for production of fermentable sugars. Maximum enzymatic hydrolysis was obtained by decreasing the crystallinity of the cellulose and hemicellulose present in the banana stem biomass with the help of different chemical pretreatments. Different physico-chemical pretreatments are in practice, among them dilute acid or alkali pretreatment using sulphuric acid or sodium hydroxide concentration below 4 % (w) was economical and providing higher hydrolysis rate with cellulase enzyme (Esteghlalian et al., 1997[7]). Comparative pretreatments have been done with sulphuric acid, sodium hydroxide, sodium hydroxide (0.5 %) catalyzed sodium sulphide and sodium sulphite to enhance the hydrolysis yield at different conditions of temperature, time and acid concentrations specified through RSM. Second order polynomial equations giving hydrolysis yield (Y, gL-1) as a function of time (hour), temperature (oC) and acid concentration (%) were obtained as: (Equation 2 for Model “a” (H2SO4), Equation 3 for Model “b” (NaOH), Equation 4 for Model “c” (NaOH catalyzed Na2S), Equation 5 for Model “d” (NaOH catalyzed Na2SO3).

Effect of acid, alkali, sulfide and sulfite on lignin removal and hemicellulose hydrolysis yield
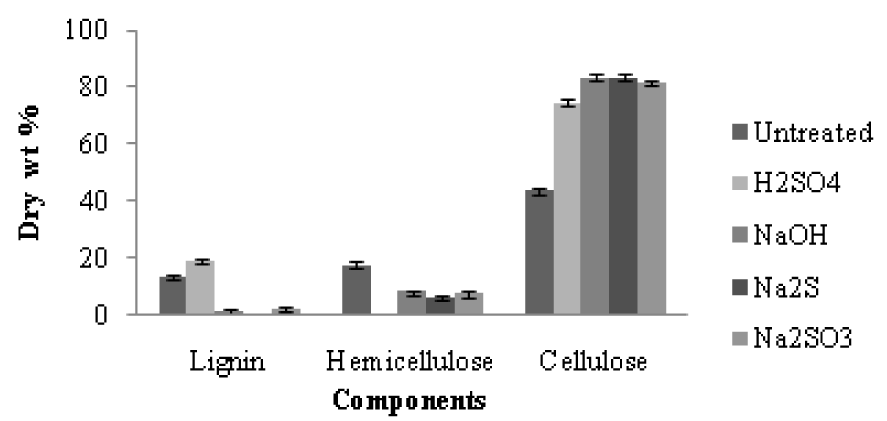
The amount of cellulosic residue after pretreatment was found to be different for different catalysts at varying conditions as shown in Table 2(Tab. 2). The sulphuric acid shows slight loss in the mass of the pseudo stem ranging from 25 % to 60 %, as compared to alkali, sulfides and sulfite which showed 5-10 % more loss during pretreatment. Hemicellulose completely hydrolyzed with sulphuric acid at higher temperature (130 °C) which was predictable and has been reported by several authors (Esteghlaian et al., 1997[7]; Lu and Mosier, 2007[22]; Kootstra et al., 2009[18]; Radecki et al., 1988[29]), with slight degradation of the resulting sugars. While NaOH, Sulfide and Sulfite hydrolyzed slight less hemicellulose as compared to acid, with complete degradation of the xylose and glucose (Figure 1, A&B(Fig. 1)). The NaOH, Na2S and Na2SO3, during pretreatment completely remove the lignin, resulting in greater than 90 % enzymatic hydrolysis yield while sulphuric acid removes about 25-35 % lignin during pretreatment (Figure 2(Fig. 2)). After removal of lignin and hydrolysis of the hemicellulose during pretreatment, the amount of the cellulose increased in the residual biomass. Sulphuric acid treated banana stem shows a lesser amount of cellulose while caustic, sulfide and sulfite shows about 8-10 % more cellulose (Figure 2(Fig. 2)). During pretreatment step with sulphuric acid as a catalyst, 33+1 gL-1 reducing sugars are obtained from hemicellulose hydrolysis which is available for fermentation (Radecki et al., 1988[29]) but no sugars were present when banana stem was treated with alkali.
Effect of time on pretreatment
The cellulosic residue and byproducts formed during pretreatment step were different for different times at different temperatures. In usual practice high temperature and long time for pretreatment hydrolyzed more hemicellulose for production of reducing sugar (Partanen and Mroz, 1999[27]). The amount of reducing sugars was less at short time at low temperature and high at higher temperature and vice versa during pretreatment (Kabel et al., 2007[16]). The quantity of reducing sugars at higher temperature and long time was less due to the degradation of the xylose and arabinose into furfural at higher temperature and long time (Masami et al., 2008[23]). Sulphuric acid with 3 % concentration produced 33.76 gL-1 and 31.01 gL-1 of reducing sugars at 130 °C when treated for one and three hour respectively. Enzymatic hydrolysis yield significantly influenced by the duration of the time for pretreatment, low acid concentration for long time of pretreatment provided higher yield, while high acid concentration with short time of pretreatment gave higher amount of sugar. Biomass treated with 1 % sulphuric acid at 130 °C produced 35.03 gL-1 and 37.67 gL-1 of reducing sugars from enzymatic hydrolysis, when treated for one and three hours respectively. When Banana stem treated with sodium hydroxide at 130 °C for 1 and 3 hours, enzymatic hydrolysis produced 39.32 gL-1 and 41.02 gL-1 of reducing sugars. Banana stem treated with sulfide and sulfite, at 130 °C for one and three hours of pretreatment produced 41 gL-1 and 40 gL-1 of reducing sugars during enzymatic hydrolysis (Table 2(Tab. 2)).
Effect of temperature on pretreatment
At low temperature conditions, the solid residue available for hydrolysis have high hemicellulose content while at high temperature maximum hemicellulose was hydrolyzed into component sugars whichever the catalyst was used for pretreatment. At high temperature and time, the maximum hydrolysis of the hemicellulose was observed in pretreatment steps which results in decreasing the remaining polysaccharides (50-60 % loss) (Table 2(Tab. 2)). During acid pretreatment, hemicellulose material was depicted to be converted into xylose and arabinose, less at lower temperature while at higher temperature complete hydrolysis was obtained with maximum amount of reducing sugars ranging from 13-33 gL-1 which is available for fermentations. Sulphuric acid (1 % conc.) produced 13.72 gL-1 and 30.39 gL-1 of reducing sugars when pretreated for one hour at 90 oC and 130 oC respectively. With the increase in temperature for pretreatment, the reducing sugars produced from hemicellulose (Satimanont et al., 2012[30]; Ezeji et al., 2007[8]; Ackerson et al., 1981[1]) were converted into furfural and 5-hydroxy furfural. When pretreatment was performed at 110 °C with sulphuric acid, no furfural was produced while at 130 °C furfural was observed at upper edge of TLC image (Figure 1(Fig. 1)). The amount of furfural depends on the acid concentration and temperature (Gonzales et al.,1986[10]). Alkali and alkali catalyzed sulfide and sulfite decrease the biomass up to 63 % by removing the lignin (<95 %) along with hydrolysis of hemicellulose at higher temperatures. Enzymatic hydrolysis of sulphuric acid treated biomass was 58.66 % and 70.06 % when pretreatment was done at 90 °C and 130 °C at same acid concentration, while sulfide shows 53.32 % and 82.8 % hydrolysis yield respectively. Temperature significantly affects the hydrolysis yield (Figure 3(Fig. 3)). At low temperature with short time of pretreatment, hydrolysis yield was less while at high temperature hydrolysis yield was greater.
Response surface for the interactions of catalyst concentration, temperature and time on hydrolysis yield
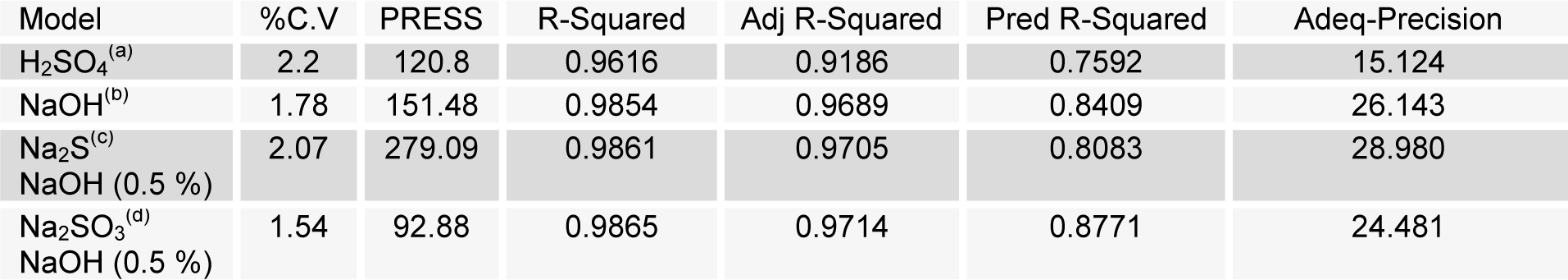
To assess the effect of different reaction parameter of pretreatment on the hydrolysis yield, quadratic models were selected out of linear, 2FI, quadratic and cubic based on sequential model testing, lack of fit test and model summary statistic (Table 3(Tab. 3)). The fitness of the model was verified through different diagnostic checks. Residuals were found to follow the normality and the plots of predicted versus actual yield (Figures 4(Fig. 4) and 5(Fig. 5)) also ascertained the overall fitness of the suggested model. Analysis of variance (ANOVA) for the quadratic models (a, b, c, d) suggested that the models were adequate to express the actual relationship between the response and significant variables, with a satisfactory coefficient of determination (adj R2= 0.91, 0.96, 0.97, 0.97 respectively), which indicated 91, 96, 97, 97 % of the variability in the response could be explained by the 2nd order polynomial predictive equations given above (1, 2, 3 & 4). Significance of linear terms i.e., catalyst concentration, time (hour) and temperature (T) for pretreatment, were found to be significant at 5 % level. Also, the P-value of the lack of fit 0.154, 0.167, 0.083, and 0.176 respectively (Table 4(Tab. 4)), confirmed that the polynomial models fit the processing.
Optimization of pretreatment factors for maximum hydrolysis yield
The main objective of this work was to find optimal conditions for the production of maximum fermentable sugars from the waste banana pseudo stem which would be used for lactic acid production. Accordingly, numerical and graphical optimization was performed with statistical tools. A variety of pretreatment conditions, with desirability of 1, were selected for maximum yield at lowest catalyst level (1 % catalyst). Moreover, when we decreased the catalyst concentration from 3 % to 1 %, while keeping temperature and time in range, we found hydrolysis yield to be in the range of 84-85 %. Although the yield decreased from 89 % to 85 % from the cost-efficiency and processing safety, we suggest using the lowest catalyst concentration. To validate optimum yield, experiments with specified conditions were performed. The optimized condition for NaOH was found to be: conc. 1 %, temperature 130 °C for 2.6 hours; Na2S: conc. 1 %, temperature 130 °C for 2.29 hours; Na2SO3: conc. 1 %, temperature 130 °C for 2.41 hours and H2SO4 conc. 1 %, temperature 129.45 °C for 2.18 hours, with 84.91 %, 85.23 %, 81.2 % and 76.02 % hydrolysis yield, respectively. Resultant hydrolysis yield showed that models were predictive and useful for the optimization of the pretreatment conditions.
Lactic acid production from enzymatic hydrolyzate
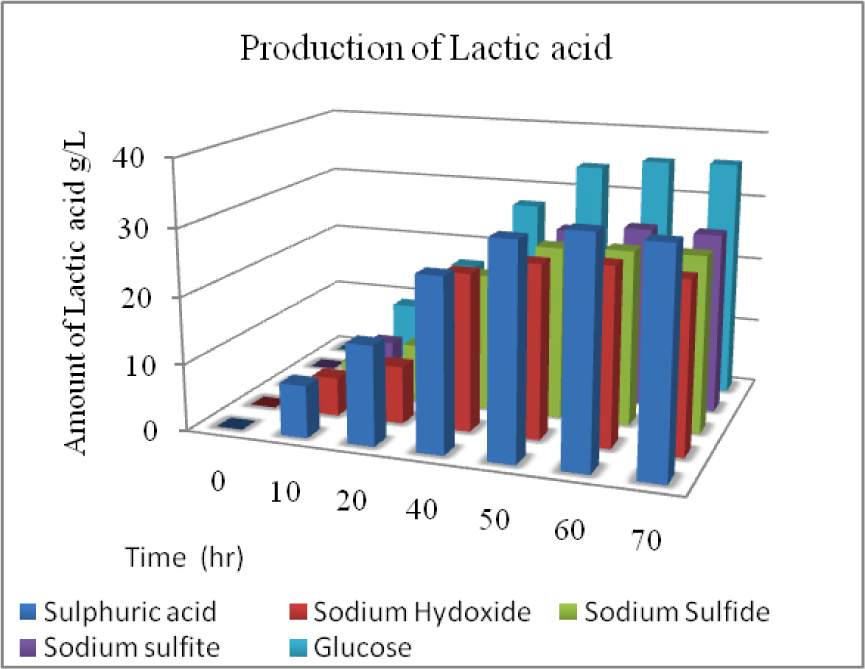
Lactic acid fermentation was conducted on the hydrolyzate that was obtained from the enzymatic hydrolysis with the most effective pretreatment conditions. The reducing sugar concentrations were adjusted at 40 gL-1 by diluting and concentrating the hydrolyzate. Lactic acid conversion yield from pure glucose and enzymatic hydrolyzate obtained from acid treated pseudo stem were greater than 90 % (Figure 6(Fig. 6)) which was comparable to the finding of Oh et al. (2005[26]) and greater than Miura et al. (2004[24]) and Moldes et al. (2006[25]). Alkali treated hydrolyzate produced less lactic acid yield, i.e., greater than 80 %. DNS analysis showed that acid treated enzymatic hydrolyzate after lactic acid fermentation showed less (3.2 gL-1) reducing sugars while alkali treated enzymatic hydrolyzate showed more sugars (7-9 gL-1) due to the presence of xylose which was not consumed by the L. acidophilus. Productivity of lactic acid from pure glucose was 0.82 gL-1.h-1 while acid treated enzymatic hydrolyzate showed (0.75 gL-1.h-1) which was greater than alkali treated hydrolyzate, 0.6 gL-1.h-1.
Conclusions
This study shows that response surface methodology is predictive in nature for optimizing the pretreatment of biomass for the production of fermentable sugars with high F and R2 values and low p-value. The enzymatic hydrolysis yield from alkali, alkali catalyzed sulfide and sulfite treated material were higher as compared to sulphuric acid but acid also provided reducing sugar (33 gL-1) during pretreatment. Collectively sulphuric acid credited higher amount of fermentable sugars (71 gL-1, pretreatment + enzymatic step) as compared to alkali, sulfide and sulfite. These sugars successfully converted into lactic acid with 92 % conversion yield. Banana pseudo stem seemed to be a wealthy source for the production of fermentable sugars and lactic acid at industrial level in near future by using acid treated enzymatic hydrolysis.
Acknowledgements
We gratefully acknowledge the financial support from Higher Education Commission, Govt. of Pakistan, Islamabad. Genencor International Inc. (Singapore) is also acknowledged for providing enzyme samples.
References
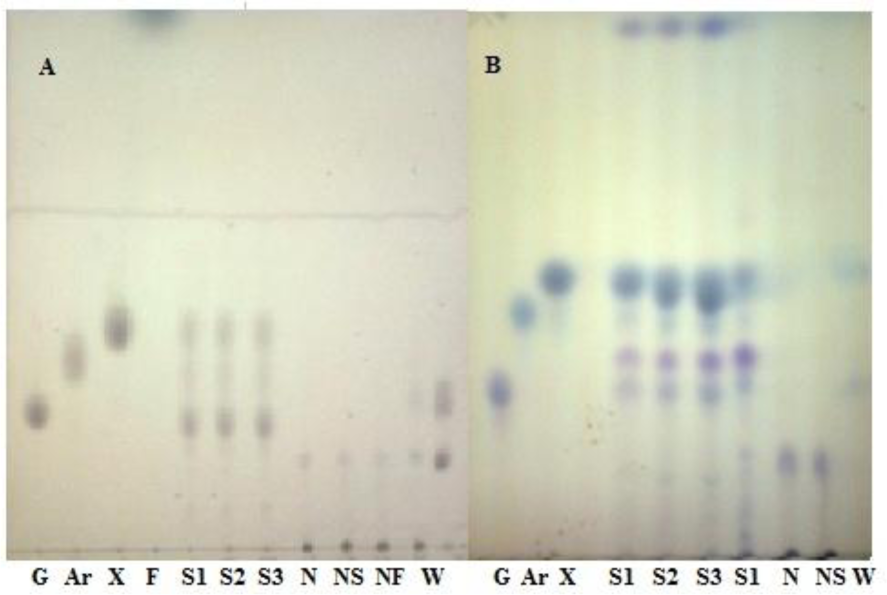
Figure 1: Monomeric sugars in pretreated hydrolyzate (A) at 110 °C for 3 hr, (B) at 130 °C for 3 hr [G: glucose, Ar: arabinose, X: xylose, F: furfural, S: sulphuric acid (S1=1 %, S2=2 %, S3=3 %), N: sodium hydroxide, NS: sodium sulfide, NF: sodium sulfite, W: water]
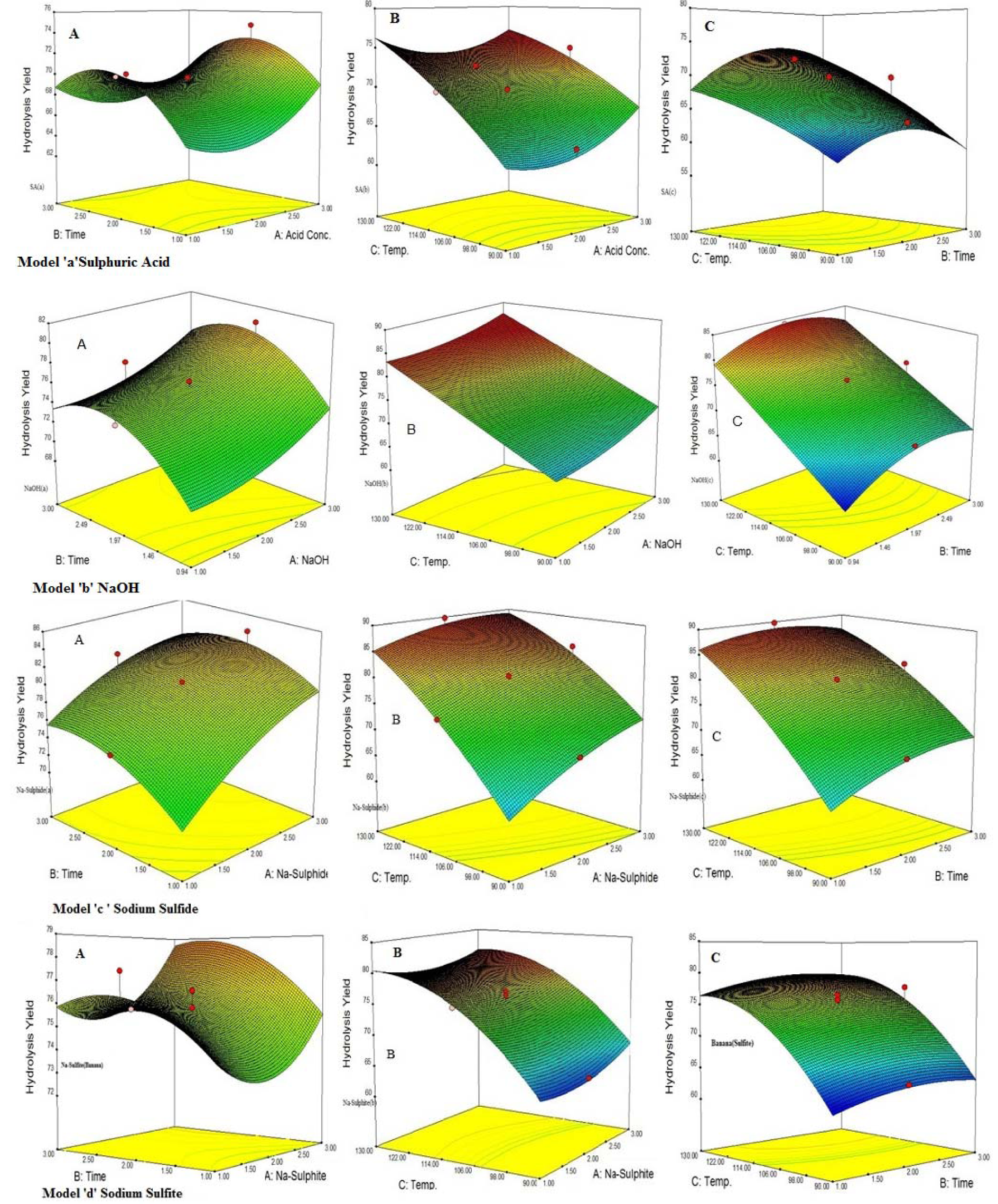
Figure 3: Interaction effect of independent variables on hydrolysis yield when H2SO4(a), NaOH (b), Na2S(c) and Na2SO3(d) were used as a catalyst for pretreatment; A (catalyst vs time), B (catalyst vs temp.) and C (time vs temp.)
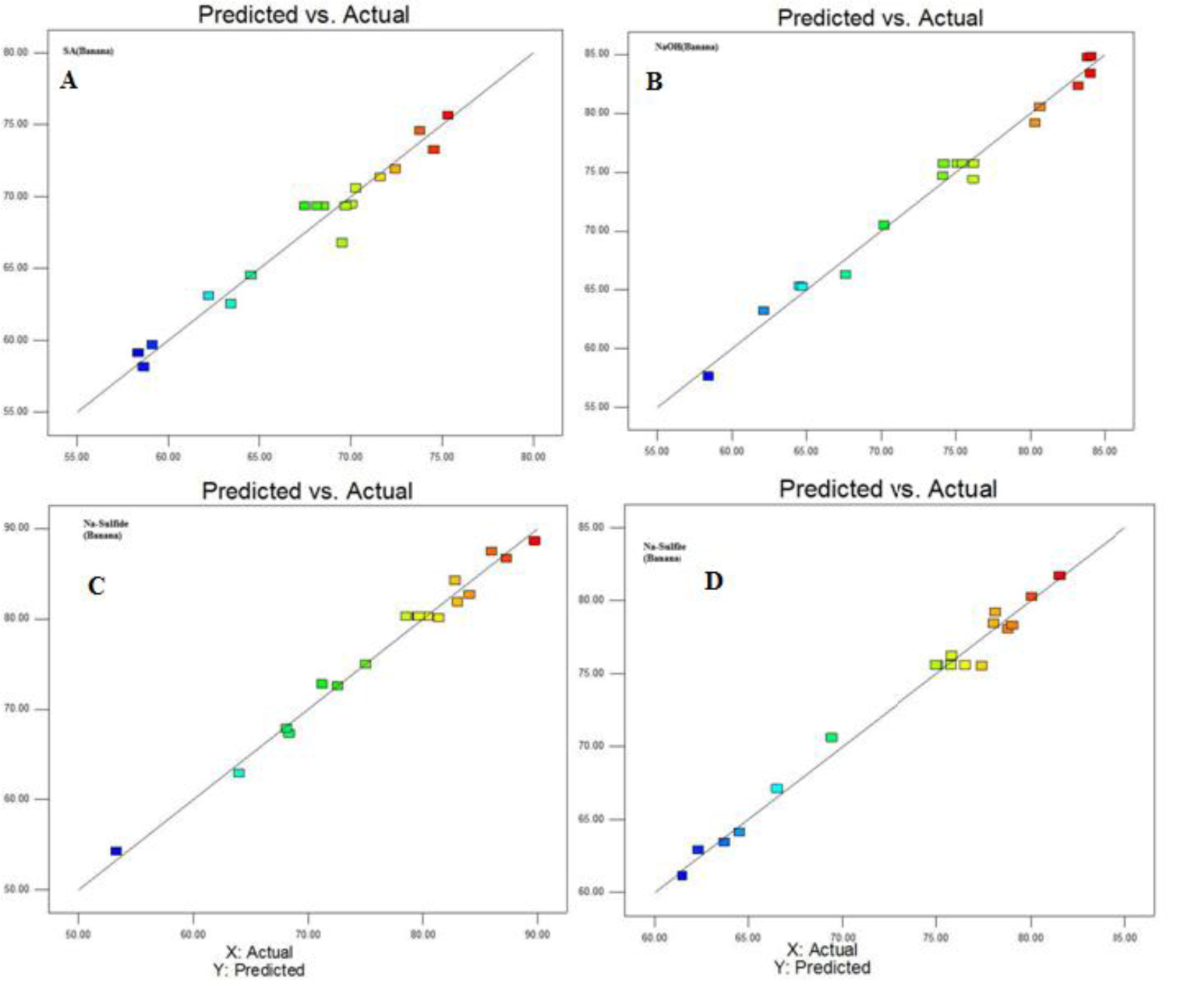
Figure 4: Plot of predicted vs actual yield when (A) H2SO4, (B) NaOH, (C) Na2S and Na2SO3 were used as a catalyst during treatment of biomass for enzymatic hydrolysis
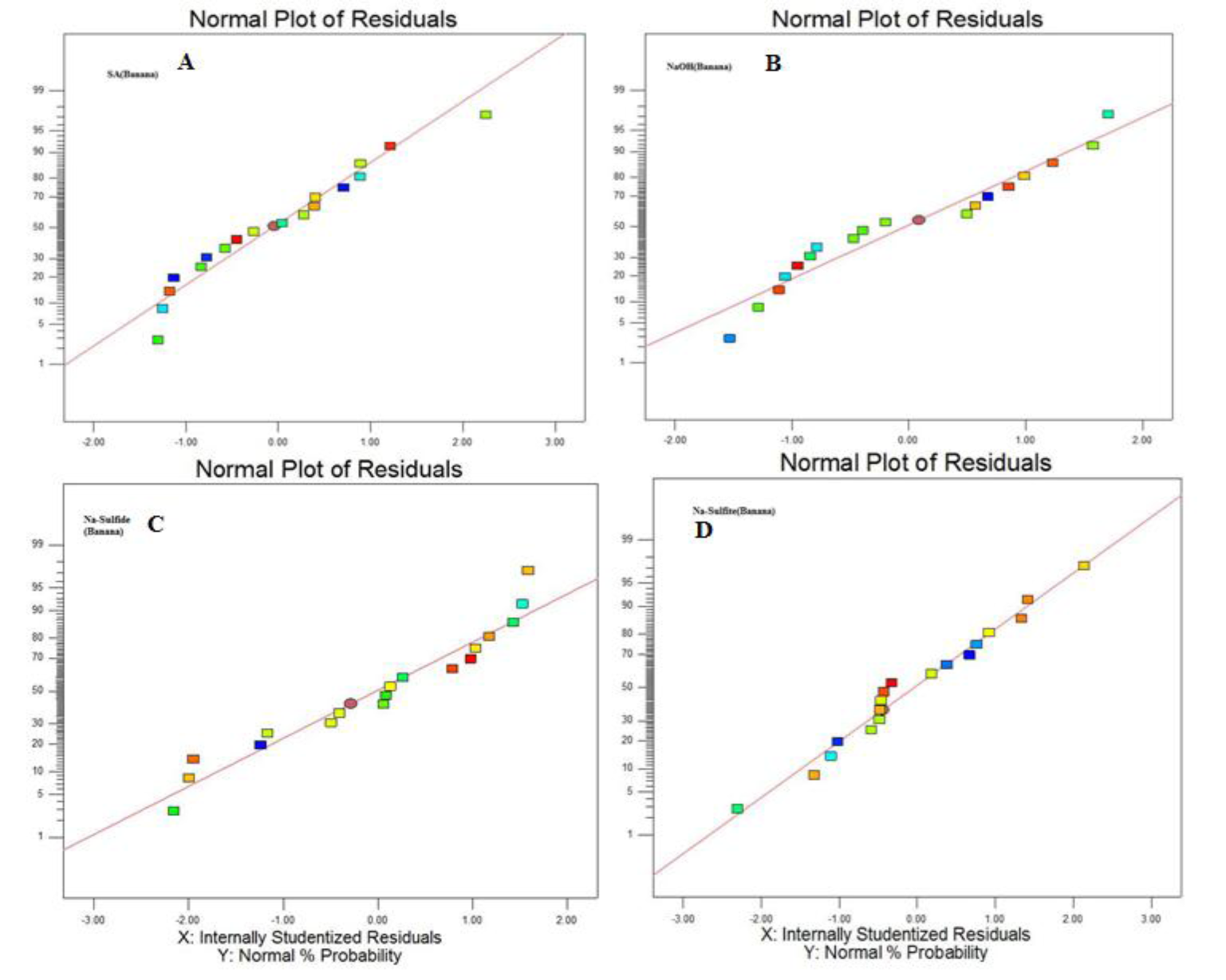
Figure 5: Normal plot of residuals vs probability when (A) H2SO4, (B) NaOH, (C) Na2S and Na2SO3 were used as a catalyst during treatment of biomass for enzymatic hydrolysis
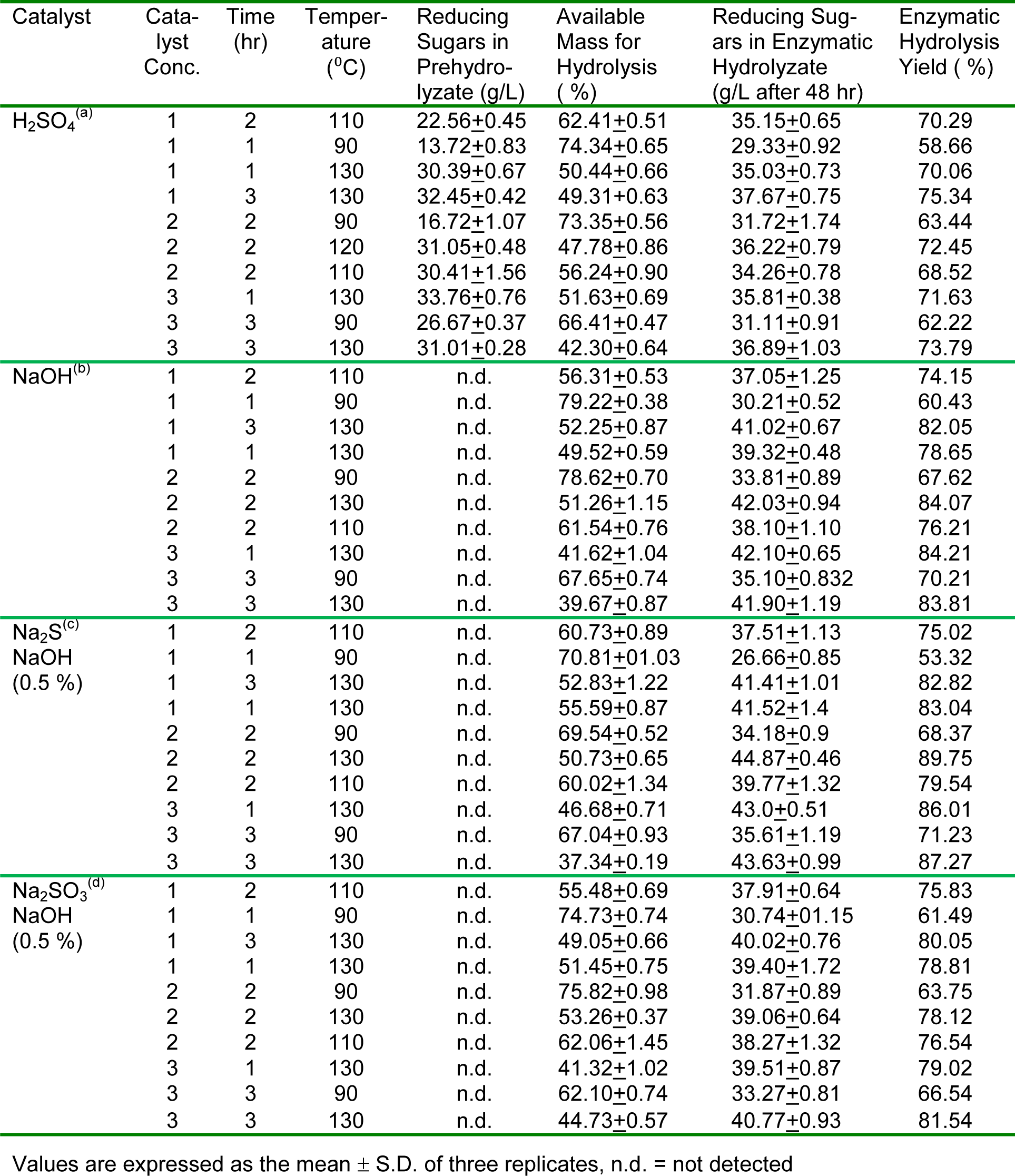
Table 2: Reaction conditions, residue in pretreatment step and reducing sugars in pretreated and enzymatic hydrolyzate
[*] Corresponding Author:
Muhammad Idrees, Department of Chemistry, GC University, Katchery Road, Lahore 54000, Pakistan; phone: +92-3214230056, eMail: m.idrees20@yahoo.com