Research article
Cannabis-induced impairment of learning and memory: effect of different nootropic drugs
Omar M.E. Abdel-Salam1[*], Neveen A. Salem1, Marwa El-Sayed El-Shamarka1, Noha Al-Said Ahmed1, Jihan Seid Hussein2, Zakaria A. El-Khyat2
1Department of Toxicology and Narcotics, National Research Centre, Cairo2Department of Medical Biochemistry, National Research Centre, Cairo
EXCLI J 2013;12:Doc193
Abstract
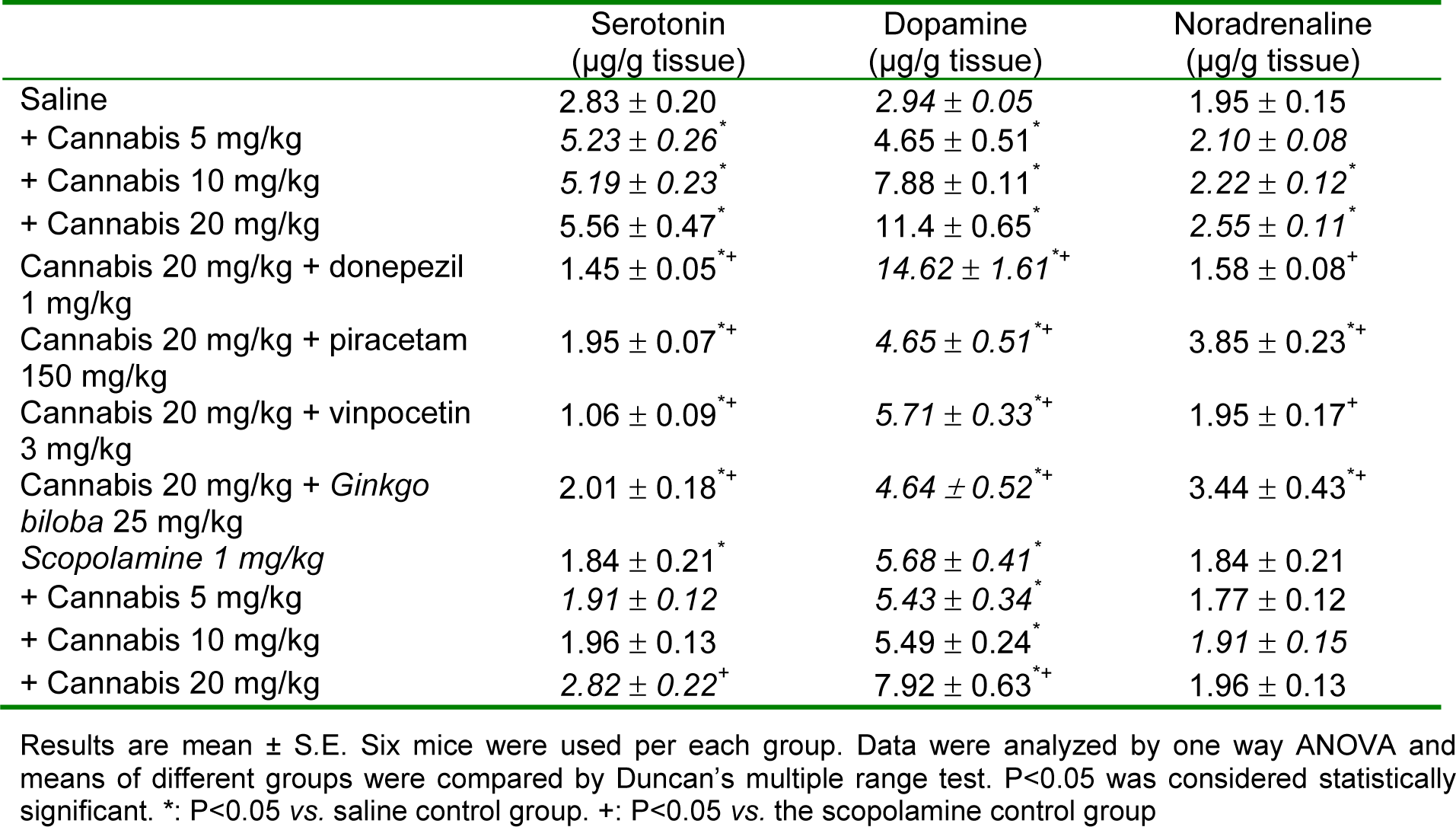
Cannabis sativa preparations are the most commonly used illicit drugs worldwide. The present study aimed to investigate the effect of Cannabis sativa extract in the working memory version of the Morris water maze (MWM; Morris, 1984[43]) test and determine the effect of standard memory enhancing drugs. Cannabis sativa was given at doses of 5, 10 or 20 mg/kg (expressed as Δ9-tetrahydrocannabinol) alone or co-administered with donepezil (1 mg/kg), piracetam (150 mg/ kg), vinpocetine (1.5 mg/kg) or ginkgo biloba (25 mg/kg) once daily subcutaneously (s.c.) for one month. Mice were examined three times weekly for their ability to locate a submerged platform. Mice were euthanized 30 days after starting cannabis injection when biochemical assays were carried out. Malondialdehyde (MDA), reduced glutathione (GSH), nitric oxide, glucose and brain monoamines were determined. Cannabis resulted in a significant increase in the time taken to locate the platform and enhanced the memory impairment produced by scopolamine. This effect of cannabis decreased by memory enhancing drugs with piracetam resulting in the most-shorter latency compared with the cannabis. Biochemically, cannabis altered the oxidative status of the brain with decreased MDA, increased GSH, but decreased nitric oxide and glucose. In cannabis-treated rats, the level of GSH in brain was increased after vinpocetine and donepezil and was markedly elevated after Ginkgo biloba. Piracetam restored the decrease in glucose and nitric oxide by cannabis. Cannabis caused dose-dependent increases of brain serotonin, noradrenaline and dopamine. After cannabis treatment, noradrenaline is restored to its normal value by donepezil, vinpocetine or Ginkgo biloba, but increased by piracetam. The level of dopamine was significantly reduced by piracetam, vinpocetine or Ginkgo biloba. These data indicate that cannabis administration is associated with impaired memory performance which is likely to involve decreased brain glucose availability as well as alterations in brain monoamine neurotransmitter levels. Piracetam is more effective in ameliorating the cognitive impairments than other nootropics by alleviating the alterations in glucose, nitric oxide and dopamine in brain.
Keywords: Cannabis sativa extract, nootropics, water maze, mice, oxidative stress, brain monoamines
Introduction
The cannabis preparations marijuana and hashish are the most popular and most commonly used illicit drugs worldwide. These are derived from the female plant of Cannabis sativa L (family Cannabinaceae). Marijuana is prepared from the dried flowering tops and leaves; hashish consists of dried cannabis resin and compressed flowers (Ashton, 2001[4]). Cannabinoids are a group of C21 terpenophenolic compounds uniquely produced by Cannabis sativa plant. The primary psychoactive constituent is Δ9-tetrahydrocannabinol (THC) (Mechoulam and Gaoni, 1967[40]). Other plant cannabinoids include Δ8-THC, cannabinol and cannabidiol (Adams and Martin, 1996[3]).
Memory or the retention of learned information is fundamental to human beings. Cannabis use causes working and short-term memory deficits in humans as well as in experimental animals (Solowij and Battisti, 2008[71]; Fadda et al., 2004[20]). This effect applies to both short and long-term use of cannabis (Solowij and Battisti, 2008[71]; Solowij et al., 2011[72]); the impairments may persist well beyond the period of intoxication, and recovery of functions might take weeks following abstinence (Pope et al., 2001[57]) or persist for longer time (Solowij et al., 2002[73]). In chronic users, cannabis might impair the ability to learn and remember new information. Early onset, long-term use and higher frequency of use are seen as risk factors for cognitive impairments (Harvey et al., 2007[27]). Cannabinoids exert their effects by interaction with specific endogenous cannabinoid receptors. Two G protein-coupled cannabinoid receptor subtypes have been cloned: CB1 receptor which is widely distributed throughout the brain, but particularly in the cerebral cortex, hippocampus, cerebellum, thalamus and basal ganglia (the brain regions involved in cognition, memory, reward, pain perception, and motor coordination) and CB2 receptor which is mainly expressed on immune cells, but also in central nervous system. These receptors also respond to endogenous ligands, the endocannabinoids such as anandamide and 2-arachidonoylglycerol, which are arachidonic acid derivatives. Cannabinoid CB1 receptors were identified in the hippocampus, the brain region most closely associated with memory (Pertwee and Ross, 2002[55]; Pertwee, 2005[53]; Svíženská et al., 2008[75]). The precise nature of memory deficits in cannabis users and their neural substrates still require further research (Solowij and Battisti, 2008[71]).
In the treatment of memory disorders whether occurring as a part of normal aging or due to a pathological process e.g., Alzheimer's disease, drugs such as piracetam, vinpocetine, Ginkgo biloba or donepezil which improve learning and memory are frequently prescribed (McDaniel et al., 2003[39]). Piracetam was the first of the so called "nootropics", a term introduced by Giurgea (1973[23]) to indicate this category of drugs that enhance memory, facilitate learning and protect memory processes against conditions which tend to disrupt them. The drug is a pyrrilodine derivative (2-oxo-1-pyrrolidine acetamide), a cyclic derivative of gamma-aminobutyric acid, which has been shown to facilitate learning and preserve memory from disruption under different experimental conditions (Gouliaev and Senning, 1994[24]; He et al., 2008[28]). Piracetam enhances recovery from post-stroke aphasia (Kessler et al., 2000[35]), improves cognitive performance in the elderly (Waegemans et al., 2002[83]) and in patients undergoing bypass surgery (Holinski et al., 2008[30]). The drug possesses rheological, blood flow promoting effects (Müller et al., 1999[45]) and mitochondrial membrane stabilizing properties (Keil et al., 2006[34]; Leuner et al., 2010[37]).
Vinpocetine (vinpocetine-ethyl apovincaminate), a synthetic derivative of the alkaloid vincamine from periwinkle (Vinca minor) is phosphodiesterase (PDE) inhibitor, selective for PDE1 (van Staveren et al., 2001[81]) and a blocker of voltage-gated Na+ channels (Sitges et al., 2006[69]) which improves learning and memory deficits in rodents (DeNoble, 1987[ref:13]). The drug is widely used to improve the cognitive functions of patients with cerebrovascular disease and chronic cerebral hypoperfusion (Horváth, 2001[ref:32]) and to decrease the risk of transient ischemic attacks and strokes in patients with chronic cerebrovascular insufficiency (Valikovics, 2007[ref:80]). Vinpocetine is a potent vasodilator at the cerebral vascular bed, increasing cerebral blood flow and regional cerebral glucose uptake (Vas et al., 2002[82]).
Standardized extracts from the leaves of Ginkgo biloba contains 24 % ginkgo-flavone glycosides and 6 % terpenoids (ginkgolides, bilobalide). Ginkgo biloba extracts are widely used to improve cognition and memory in cerebral insufficiency and mild cognitive impairment (Bäurle et al., 2009[5]). The extract also showed a beneficial effect in reducing the total and negative symptoms of chronic schizophrenia when used as an add-on therapy to antipsychotic medication (Singh et al., 2010[68]). Ginkgo biloba prevented stress- and corticosterone-induced impairments of spatial memory and reduced neuronal loss in hippocampus (Takuma et al., 2007[76]; Walesiuk and Braszko, 2009[84]). Ginkgo biloba also showed neuroprotective and therapeutic effects in experimental cerebral ischemia (Saleem et al., 2008[64]) and attenuated the toxin-induced neurodegeneration of the nigrastriatal pathway (Rojas et al., 2008[60]). The extract has been shown to possess vasodilator and blood flow enhancing (Chung et al., 1999[10]) as well as antioxidant and free radical scavenging properties (Rojas et al., 2008[60]).
The main neurochemical deficit seen in Alzheimer's disease is reduced acetylcholine content and choline acetyltransferase activity (the enzyme synthesizing acetylcholine) in the nucleus basalis of Meynert and the hippocampus (Whitehouse et al., 1982[85]). Acetylcholinesterase inhibitors (AChEI) e.g., donepezil are thus being used for the symptomatic treatment of Alzheimer's disease to enhance cholinergic neurotransmission indirectly, by inhibiting the enzyme which hydrolyses acetylcholine (Musiał et al., 2007[48]).
The aims of the present study were therefore to: (1) study the behavioral alterations associated with long-term administration of cannabis preparations on spatial learning and memory; (2) investigate the effect of memory enhancing drugs piracetam, vinpocetine, Ginkgo biloba and the acetylcholine esterase inhibitor donepezil for their possible modulation of the cannabis-induced memory alterations; (3) detect alterations in the level of the brain neurotransmitters dopamine, serotonin (5-HT) and noradrenaline; (4) assess the effect of cannabis administration on oxidative stress markers in brain since oxidative stress has been implicated in memory impairment (Clausen et al., 2010[11]). In addition, the effect of cannabis on liver oxidative stress and liver enzymes under the different experimental conditions was studied. A total extract from Cannabis sativa was used based on the fact that the effect of the whole plant which is abused by humans differs from that of THC in view of its content of other cannabinoids, terpenoids and flavonoids (Russo and McPartland, 2003[63]).
Materials and Methods
Animals
Swiss male albino mice 20-22 g of body weight were used. Standard laboratory food and water were provided ad libitum. Animal procedures were performed in accordance with the Ethics Committee of the National Research Centre and followed the recommendations of the National Institutes of Health Guide for Care and Use of Laboratory Animals (Publication No. 85-23, revised 1985). Equal groups of 6 mice each were used in all experiments.
Drugs
Vinpocetine (Vinporal, Amrya. Pharm. Ind., Cairo, ARE), piracetam (Nootropil, Chemical Industries Development; CID, Cairo, ARE), Ginkgo biloba (EMA Pharm. Co., Cairo, A.R.E) and scopolamine (Sigma, USA) were used. All drugs were dissolved in isotonic (0.9 % NaCl) saline solution immediately before use. The doses of drugs used in the study were based upon the human dose after conversion to that of mice according to Paget and Barnes conversion tables (1964[51]). Cannabis sativa L. plant was supplied by the Ministry of Justice - Egypt.
Preparation of cannabis extract
Cannabis sativa extract was prepared from the dried flowering tops and leaves of the plant. The method of extraction followed that described by Turner and Mahlberg (1984[79]) with modification as described elsewhere (Abdel-Salam et al., 2012[1]). Tetrahydrocannabinol (THC) content was quantified using GC mass. The Δ9-THC content of the extract was 10 %. The extract was injected intraperitoneally at doses of 5, 10 or 20 mg/kg (expressed as Δ9-THC). The injection volume was 0.2 ml/mice.
Cognitive testing
The maze consisted of a glass tank, narrowed to 20 cm wide, 40 cm in height, 70 cm in length, filled to a depth of 21 cm with water maintained at 25 °C. The escape glass platform was hidden from sight, submerged 1 cm below the surface of the water at the end of the tank (Dunnett et al., 2003[16]). The effect of cannabis extract was studied in normal mice and in mice treated with scopolamine (1 mg/kg, i.p.) to induce cognitive impairment (Smith et al., 2002[70]). Mice were treated with scopolamine alone or in combination with cannabis (20 mg/kg, s.c.) (n = 6/group) 30 min prior to testing. In addition, the effect of cannabis extract (20 mg/kg, s.c.) was studied in mice treated with memory enhancing drugs piracetam (150 mg/kg, s.c.), vinpocetine (1.5 mg/kg, s.c.), Ginkgo biloba (25 mg/kg, s.c.) and the acetylcholinesterase inhibitor donepezil (1 mg/kg, s.c.) for their possible modulation of the Cannabis sativa-induced memory alterations. Treatments were given once daily for 30 days. Each test consisted of three trials involving placing the mouse in the water maze with the platform hidden until it finds the platform, leaving it on the platform for 15 s, and then retesting the mouse from the same start position 3 min later (trial 1; reference memory or acquisition trial; trials 2 & 3; working memory or retrieval trials). This was done 3 times a week for 4 weeks. At the end of each trial, the mouse was towel dried, returned to its home cage (where a heat lamp was available). The latency to find the platform (s) is assessed with a stopwatch.
Biochemical studies
At the end of the study, mice were euthanized by decapitation, brains and livers were then removed, washed with ice-cold saline solution (0.9 % NaCl), weighed and stored at -80 ºC for the biochemical analyses. The tissues were homogenized with 0.1 M phosphate buffer saline at pH 7.4, to give a final concentration of 10 % w/v for the biochemical assays. For the determination of monoamine neurotransmitters, frozen brain samples were homogenized in cold 0.1 N-perchloric acid.
Determination of lipid peroxidation
Lipid peroxidation was assayed in brain and liver homogenates by measuring the level of malondialdehyde (MDA). Malondialdehyde was determined by measuring thiobarbituric reactive species using the method of Ruiz-Larrea et al. (1994[62]) in which the thiobarbituric acid reactive substances react with thiobarbituric acid to produce a red colored complex having peak absorbance at 532 nm.
Determination of reduced glutathione
Reduced glutathione (GSH) was determined by Ellman's method (1959[19]). The procedure is based on the reduction of Ellman´s reagent by -SH groups of GSH to form 2-nitro-s-mercaptobenzoic acid, the nitromercaptobenzoic acid anion has an intense yellow color which can be determined spectrophotometrically.
Determination of nitric oxide
Nitric oxide measured as nitrite was determined by using Griess reagent, according to the method of Moshage et al. (1995[44]), where nitrite, stable end product of nitric oxide radical, is mostly used as indicator for the production of nitric oxide.
Determination of liver enzymes
Aspartate aminotransferase (AST) and alanine aminotransferase (ALT) activities in liver were measured according to Reitman-Frankel colorimetric transaminase procedure (Crowley, 1967[12]) using commercially available kits (BioMérieux, France).
Determination of brain glucose
Brain tissue glucose content was determined according to the method of Trinder (1969[78]). Glucose in the presence of glucose oxidase is converted to peroxide and gluconic acid. The produced hydrogen peroxide reacts with phenol and 4-amino-antipyrine in the presence of peroxidase to yield a colored quinonemine, which is measured spectrophotometrically.
Determination of brain monoamines
Determination of brain serotonin, noradrenaline and dopamine was carried out using high performance liquid chromatography (HPLC) system, Agilent technologies 1100 series, equipped with a quaternary pump (Quat pump, G131A model). Separation was achieved on ODS reversed phase column (C18, 25 x 0.46 cm i.d. 5 µm). The mobile phase consisted of potassium phosphate buffer/methanol 97/3 (v/v) and was delivered at a flow rate of 1 ml/min. UV detection was performed at 270 nm and the injection volume was 20 µl. The concentration of both catecholamines and serotonin were determined by external standard method using peak areas. Serial dilutions of standards were injected and their peak areas were determined. A linear standard curve was constructed by plotting peak areas versus the corresponding concentrations. The concentration in samples was obtained from the curve.
Statistical analysis
Data are expressed as mean ± SEM. The data were analyzed by one way ANOVA and by repeated measures ANOVA, followed by Duncan's multiple range test, using SPSS software (SAS Institute Inc., Cary, NC). A probability value of less than 0.05 was considered statistically significant.
Results
Behavioral testing
Spatial memory
Cannabis substantially impaired water maze performance. The time taken to find the escape platform (latency) was significantly delayed by cannabis in a dose-dependent manner, compared with the saline-treated control group (Figure 1(Fig. 1)). The average mean latency and standard error of the mean over 4 weeks for the saline, cannabis 5 mg/kg, cannabis 10 mg/kg and cannabis 20 mg/kg was 3.19 ± 0.07, 4.78 ± 0.23, 5.73 ± 0.42 and 7.62 ± 0.52 sec, respectively. There was a significant main drug effect (F = 36.63, p = 0.001), a significant main effect of days (F = 8.82, p = 0.001) but no significant main effect for trials (F = 1.62, p = 0.207). There was a significant drug x days interaction (F = 1.76, p = 0.006) but no significant drug x trial (F = 0.33, p = 0.92) or trial x days interaction (F = 1.25, p = 0.20).
Donepezil, piracetam, vinpocetine or Ginkgo biloba co-administered with cannabis (20 mg/kg) resulted in significantly shorter latencies compared with the cannabis (20 mg/kg) only-treated group, which indicated improved learning and memory (Figure 2(Fig. 2)). The average mean latency and standard error of the mean over 4 weeks for the cannabis 20 mg/kg plus donepezil, piracetam, vinpocetine or Ginkgo biloba was 4.87 ± 0.21, 4.42 ± 0.19, 5.18 ± 0.28 and 4.65 ± 0.23 sec, respectively compared to 7.62 ± 0.52 sec for the cannabis 20 mg/kg group (Figure 3(Fig. 3)). Compared with the cannabis only group, the escape latency decreased by 36.1 %, 42 %, 32 % and 39 % by donepezil, piracetam, vinpocetine or Ginkgo biloba, respectively. Piracetam resulted in significantly shorter latency compared with vinpocetine by 14.7 %. There was a significant main drug effect (F = 14.45, p = 0.001), a significant main effect of days (F = 5.27, p = 0.001), no significant main effect of trials (F = 3.42, p = 0.038). There was a significant drug x days interaction (F = 3.19, p = 0.001) but no significant drug x trial (F = 0.38, p = 0.93) or trial x days interaction (F = 1.4, p = 0.11).
In the first trial, the time taken to find the escape platform was significantly delayed by Cannabis sativa (Figure 4A(Fig. 4)). There was a significant main effect of drug (F = 18.61, p = 0.001), a significant main effect of days (F = 2.18, p = 0.017) but no significant drug x days interaction (F = 0.86, p = 0.69). Mice treated with cannabis 20 mg/kg in combination with donepezil, piracetam, vinpocetine or Ginkgo biloba exhibited significantly shorter escape latencies compared with cannabis (20 mg/kg) only-treated group, suggesting improvement of the cognitive impairing effects of cannabis by these drugs. There was a significant main effect of drug (F = 5.0, p = 0.004), a significant main effect of days (F = 2.09, p = 0.021) but no significant drug x days interaction (F = 0.97, p = 0.54). The average mean for the saline, cannabis 5 mg/kg, cannabis 10 mg/kg and cannabis 20 mg/kg was 3.33 ± 0.14, 5.24 ± 0.47, 6.02 ± 0.45, 8.27 ± 0.44 sec, respectively. The average mean latency and standard error of the mean over 4 weeks for the cannabis 20 mg/kg plus donepezil, piracetam, vinpocetine or Ginkgo biloba was 5.64 ± 0.46, 5.11 ± 0.47, 5.54 ± 0.42 and 5.08 ± 0.43 sec, respectively (Figure 5A(Fig. 5)). Compared with the cannabis only group, the escape latency decreased by 31.8 %, 38.2 %, 33 % and 38.6 % by donepezil, piracetam, vinpocetine or Ginkgo biloba, respectively. No significant differences were observed between the different drugs in trial 1.
In the second trial, mice that received 10 or 20 mg/kg of cannabis were also delayed compared with the saline-treated group in escaping from the water maze (Figure 5B(Fig. 5)). There was a significant main effect of drug (F = 14.89, p = 0.001), a significant main effect of days (F = 3.86, P= 0.001) but no significant drug x days interaction (F = 1.13, p = 0.29). Donepezil, piracetam, vinpocetine or Ginkgo biloba co-administered with cannabis (20 mg/kg) improved performance resulting in significantly shorter latencies to find the hidden platform than the cannabis (20 mg/kg) only-treated group (Figure 5B(Fig. 5)). There was a significant main effect of drug (F = 4.73, P = 0.006), a significant main effect of days (F = 2.76, p = 0.002) and a significant drug x days interaction (F = 1.95, p = 0.001). The average mean for the saline, cannabis 5 mg/kg, cannabis 10 mg/kg and cannabis 20 mg/kg was 3.15 ± 0.11, 4.38 ± 0.36, 5.33 ± 0.43 and 7.57 ± 0.50 sec, respectively. The average mean latency and standard error of the mean over 4 weeks for the cannabis 20 mg/kg plus donepezil, piracetam, vinpocetine or Ginkgo biloba was 4.39 ± 0.28, 4.22 ± 0.29, 5.09 ± 0.32 and 4.98 ± 0.21 sec, respectively. Compared with the cannabis only group, the escape latency decreased by 42 %, 44.2 %, 32.8 % and 34.2 % by donepezil, piracetam, vinpocetine or Ginkgo biloba, respectively. Piracetam resulted in significantly shorter latency compared with vinpocetine by 17.1 %.
In the third trial, mice that received cannabis took significantly more time to find the escape platform compared with the saline-treated group (Figure 5C(Fig. 5)). There was a significant main effect of drug (F = 7.36, p = 0.002), a significant main effect of days (F = 5.12, p = 0.001) but no significant drug x days interaction (F = 0.96, P = 0.53). Donepezil, piracetam, vinpocetine or Ginkgo biloba co-administered with cannabis (20 mg/kg) resulted in significantly shorter latencies compared with cannabis (20 mg/ kg) only-treated group (Figure 5C(Fig. 5)). There was a significant main effect of drug (F = 5.60, P = 0.002), a significant main effect of days (F = 3.65, p = 0.0001) and a significant drug x days interaction (F = 2.0, p = 0.001). The average mean for the saline, cannabis 5 mg/ kg, cannabis 10 mg/kg and cannabis 20 mg/ kg was 3.06 ± 0.1, 4.71 ± 0.36, 5.85 ± 0.41 and 7.02 ± 0.60 sec, respectively. The average mean latency and standard error of the mean over 4 weeks for the cannabis 20 mg/ kg plus donepezil, piracetam, vinpocetine or Ginkgo biloba was 4.95 ± 0.31, 3.94 ± 0.20, 4.91 ± 0.26 and 3.89 ± 0.19 sec, respectively. Compared with the cannabis only group, the escape latency decreased by 29.4 %, 43.9 %, 30.1 % and 44.6 % by donepezil, piracetam, vinpocetine or ginkgo biloba, respectively. Piracetam or Ginkgo biloba resulted in significantly shorter latency compared with vinpocetine by 19.7 % and 20.1 % and compared with donepezil by 20.4 %, 21.4 %, respectively.
Scopolamine (1 mg/kg, i.p.) substantially impaired cognitive performance leading to higher latencies to locate the submerged platform compared with the saline-treated group. Mice treated with scopolamine and cannabis (10 or 20 mg/kg, sc) needed significantly more time to locate the hidden platform than the scopolamine only-treated group, suggesting enhancement of the cognitive impairing effects of scopolamine by cannabis (Figures 6(Fig. 6), 7(Fig. 7)) (2-way repeated measures ANOVA; drug effect: F = 2.19, p = 0.09). There was no significant effect of trials (F = 1.71, p = 0.19) and no significant main effect of days (F = 0.89, p = 0.55). There was a significant drug x days interaction (F = 1.69, P = 0.01) but no significant drug x trial (F = 0.37, p = 0.89) or trial x days interaction (F = 0.83, p = 0.69). The average mean latency and standard error of the mean over 4 weeks for the saline, scopolamine 1 mg/kg and the scopolamine plus cannabis at 5, 10 or 20 mg/kg was 3.13 ± 0.06, 6.92 ± 0.35, 6.32 ± 0.23, 8.07 ± 0.47 and 9.04 ± 0.63 sec, respectively. The time taken to find the escape platform was significantly delayed by scopolamine alone or in combination with cannabis compared with the saline treated group in the first, second and third trial (2-way repeated measure ANOVA: drug effect: trial 1: F = 1.11, p = 0.37; trial 2: F = 0.5, p = 0.69; trial 3: F = 1.22, p = 0.33). There was no significant main effect of days on the escape latency and no significant drug x days interaction in all trials (data not shown). In the second trial, mice treated with scopolamine + 20 mg/kg cannabis showed significantly higher latencies to find the hidden platform compared with scopolamine + 5 mg/kg cannabis-treated group. In the third trial mice that received scopolamine + 20 mg/kg cannabis showed significantly higher latencies to find the submerged platform compared with other groups (Figure 4A-C(Fig. 4)).
Biochemical results
Effect of cannabis on markers of oxidative stress and glucose in brain
Cannabis alone
The administration of cannabis altered the redox status in brain with the effect being significant with the high dose of the extract which resulted in 20.2 % decrease in MDA compared with the saline-treated group (26.71 ± 0.96 vs. 33.47 ± 1.34 nmol/g). Brain GSH showed significant increase by 24.2 % after cannabis at 20 mg/kg (4.1 ± 0.20 vs. 3.3 ± 0.13 µmol/g, p<0.05). Meanwhile, the administration of cannabis at 20 mg/kg resulted in a significant decrease in brain nitrite by 32.8 % (34.48 ± 3.3 vs. 51.3 ± 3.1 µmol/g, p<0.05) and in brain glucose by 30.2 % (35.1 ± 1.9 vs. 50.3 ± 3.5 µg/g, p<0.05) (Figure 8 A-D(Fig. 8)).
Cannabis in combination with memory enhancing drugs
The level of MDA in brain increased significantly by 23.8 % after the administration of piracetam (33.08 ± 0.76 vs. 26.71 ± 0.96 nmol/g, p<0.05). Meanwhile, brain nitrite and glucose which decreased by cannabis were restored to near normal values by piracetam, registering 29.1% (44.5 ± 2.9 vs. 34.48 ± 3.3 µmol/g, p<0.05) and 36.2 % (47.8 ± 2.0 vs. 35.1 ± 1.9 µg/g, p<0.05) increments compared with the 20 mg/kg cannabis only-treated group. On the other hand, the administration of donepezil, vinpocetine or Ginkgo biloba resulted in significant increase in brain GSH by 36.6 %, 41.5 % and 117.1 %, respectively (Figure 8A-D(Fig. 8))
Cannabis in combination with scopolamine
The administration of scopolamine at the dose of 1 mg/kg did not change brain MDA or glucose level. However, the level of nitric oxide increased by 15.7 % and GSH significantly decreased by 27.3 % by scopolamine vs. saline control value (2.4 ± 0.12 vs. 3.3 ± 0.13 µmol/g, p<0.05). In scopolamine-treated mice, the administration of cannabis extract decreased MDA in a dose-dependent manner. A significant decrease in MDA level by 26.2 % and 31.7 % was observed after the administration of cannabis extract 10 or 20 mg/kg, respectively (27.87 ± 1.04 and 25.76 ± 0.98 vs. 37.74 ± 1.23 nmol/g, p<0.05). The level of glutathione was significantly increased by 29.2 % (p<0.05) and 62.5 % (p<0.05) after the administration of cannabis extract at the doses of 10 and 20 mg/kg, respectively, compared with the scopolamine only-treated group (3.1 ± 0.0.16 and 3.9 ± 0.26 vs. 2.4 ± 0.12 µmol/g, p<0.05). The level of nitrite significantly decreased by 26.3 % after cannabis at 20 mg/kg, while glucose level significantly decreased by 16.7 % and 30.6 % after cannabis at 10 and 20 mg/kg, respectively vs. scopolamine only-treated group (Figure 8 A-D(Fig. 8)).
Effect of cannabis on brain monoamines
In saline-treated mice, a dose-dependent elevation of serotonin, noradrenaline and dopamine was observed after treatment with cannabis extract alone at tested doses of 5, 10 and 20 mg/kg. In mice given 20 mg/kg of cannabis, the increment in serotonin was ameliorated by the memory enhancing drugs donepezil, piracetam, vinpocetine or Ginkgo biloba. Noradrenaline was restored to its normal value by donepezil, vinpocetine or ginkgo biloba, but increased by piracetam. The level of dopamine was significantly reduced by piracetam, vinpocetine or Ginkgo biloba, but not by donepezil (Table 1(Tab. 1)). In mice treated with scopolamine, a significant increase in serotonin and dopamine by cannabis at 20 mg/ kg was observed.
Effect of cannabis on markers of oxidative stress in liver
The effect of cannabis on oxidative markers in the liver was also examined. The administration of cannabis extract (5, 10 or 20 mg/kg) did not alter the level of MDA, GSH or nitrite in hepatic tissue. In mice given 20 mg/kg cannabis extract, the administration of Ginkgo biloba significantly decreased nitrite level (85.0 ± 5.1 vs. 102.4 ± 6.1 µmol/g, p<0.05). Meanwhile, GSH showed further increase by 17.2% in mice treated with Ginkgo biloba (12.9 ± 1.1 vs. 11.01 ± 3.1 µmol/g, p<0.05). Liver alanine aminotransferase and aspartate aminotransferase were not altered in mice given 20 mg/kg cannabis alone or with memory enhancing drugs (Table 2(Tab. 2)).
The administration of scopolamine did not alter liver malondialdehyde or nitric oxide level. However, the level of GSH was significantly decreased by 31.7 % (p<0.05) by scopolamine vs. saline control value (7.65 ± 1.3 vs. 11.2 ± 2.4 µmol/g, p<0.05). In scopolamine-treated mice, the administration of cannabis at 5, 10 or 20 mg/kg increased liver GSH in a dose-dependent manner (33.3 %, 38.6 % and 50.3 % increase vs. scopolamine only-treated group) (Figure 9 A-C(Fig. 9)).
Discussion
In the present study the effect of cannabis extract with known Δ9-THC content on working memory and on oxidative stress in brain and liver of mice was investigated. The repeated daily administration of the extract at doses corresponding to 5, 10 and 20 mg Δ9-THC/kg was associated with impaired learning and memory when tested in the water maze test. Mice given cannabis spent more time to locate the hidden platform than their untreated counterparts. The effect was most evident with the initial administration of the extract and maintained throughout the study. There was a clear dose-related response during the three trials of the test. Scopolamine, an anticholinergic drug, impaired memory performance in the water maze test which is in accordance with other studies (Smith et al., 2002[70]). Mice treated with scopolamine and cannabis needed significantly more time to locate the hidden platform than the scopolamine only-treated group, suggesting exacerbation of the cognitive impairing effects of scopolamine by cannabis. Studies have shown that cannabis preparations or their primary psychoactive constituent Δ9-THC affect memory processing in humans and in experimental animals. Prose recall and source memory were poorer in daily users with high THC levels in hair (Morgan et al., 2011[42]). In healthy volunteers, Δ9-THC (2.5, and 5 mg) given intravenously disrupted immediate and delayed word recall, impaired performance tests of distractibility, verbal fluency and working memory (D'Souza et al., 2004[15]). Similarly, orally given Δ9-THC led to acute impairment of attentional functioning and working memory (Roser et al., 2008[ref:61]). Impaired memory performance has been reported in mice after the oral administration of hashish extract in dosages corresponding to 1, 5, and 10 mg Δ9-THC/kg (Frischknecht et al., 1985[22]) and after inhalation of marijuana smoke (Niyuhire et al., 2007[49]) and in rats following intraperitoneal injection of Δ9-THC-rich extracts (2 or 5 mg/kg) (Fadda et al., 2004[20]). Cannabidiol, another constituent of the plant Cannabis sativa, that does not possess psychoactive properties, appeared to antagonize the memory impairing effect of Δ9-THC in the same extract (Fadda et al., 2004[20]), though not the working memory deficits induced by scopolamine and dizocilpine (Fadda et al., 2006[21]). The cannabis induced memory alterations are likely to be mediated by Δ9-THC-inducecd activation of cannabinoid CB1 receptor in brain. Delta (9)-THC-rich extracts impaired memory performance in rats (Fadda et al., 2004[20]), while Δ9-THC-induced spatial memory impairment was reversed by cannabinoid CB(1) receptor antagonist, suggesting that the effect of Δ9-THC is mediated through cannabinoid CB(1) receptors (Egashira et al., 2012[18]). Studies also suggested the involvement of prefrontal dopamine receptors (Rodrigues et al., 2011[59]) as well as μ- and κ-opioid receptors (Egashira et al., 2012[18]) in Δ9-THC-induced disruption of spatial working memory. Cannabis effect on learning and memory processes probably involves depolarization-induced suppression of excitatory mechanism in the CA1 area of hippocampus (Ebrahimpour et al., 2010[17]).
Of the important findings in the present studies is the effect of cannabis on neurotransmitter levels in brain. It is evident that the repeated administration of cannabis extract increased brain levels of serotonin, noradrenaline and dopamine. Other studies have shown increased dopamine and noradrenaline release in rodent brain by THC in several regions of the brain, including striatal, nucleus accumbens and prefrontal areas (Muntoni et al., 2006[46]; Robledo et al., 2007[58]). Moreover, increased release of norepinephrine in the rat frontal cortex was observed after systemic administration of the synthetic cannabinoid agonist WIN 55,212-2 (Oropeza et al., 2005[50]). In healthy human subjects, THC induced dopamine release in the human striatum (Bossong et al., 2009[7]). As for 5HT, Δ9-THC attenuated methylenedioxymethamphetamine (MDMA)-induced decreases in 5-HT levels and in serotonin transporter (SERT) binding in the frontal cortex, parietal cortex, and striatum (Shen et al., 2011[66]) while stimulating CB-1 decreased the effect of citalopram on increasing serotonin levels in the prefrontal cortex (Kleijn et al., 2011[36]). In contrast, rats injected with the synthetic cannabinoid HU210 exhibited increased 5HT1A receptor density and mRNA expression in the CA1 region of the hippocampus (Zavitsanou et al., 2010[88]). These effects of cannabis on brain monoamines could account for the cognitive and attention deficits and anxiety reactions seen in cannabis users. Cannabis sativa, however, contains many constituents including over 70 different cannabinoids (Hollister, 1988[31]). Thus cannabis can result in different effects from those of Δ9-THC alone. For example, cannabigerol a non-psychoactive constituent behaved as a potent alpha (2) adrenoceptor agonist and a 5HT(1A) antagonist (Cascio et al., 2010[9]). Meanwhile, cannabidiol, another non-psychoactive increased extracellular dopamine levels in nucleus accumbens (Murillo-Rodríguez et al., 2011[47]). While Δ9-THC acts as a CB1 and CB2 receptor partial agonist, Δ9-tetrahydrocannabivarin, behaves either as a CB1 antagonist or, at higher doses, as a CB1 receptor agonist (Pertwee, 2008[54]). The present study has also provided evidence that the nootropic drugs piracetam, vinpocetine, Ginkgo biloba or donepezil were capable of modulating neurotransmitter levels in mice treated with cannabis. Serotonin was decreased by all drugs. Noradrenaline was normalized by vinpocetine and donepezil though increased by piracetam and Ginkgo biloba. Meanwhile, piracetam, vinpocetine, Ginkgo biloba decreased the cannabis-induced increments in dopamine. Studies have shown that most nootropics influencing cognitive mechanisms affect neurotransmitters levels in brain. Vinpocetine through selective blockade of voltage-sensitive presynaptic Na+ channels inhibits the transporter-mediated release of monoamine neurotransmitters. The drug impairs the vesicular storage of dopamine as well (Trejo et al., 2001[77]). Ginkgo biloba has been shown to increase central dopamine, noradrenaline (Yoshitake et al., 2010[87]) and 5-HT (Blecharz-Klin et al., 2009[6]) levels. Piracetam increases dopamine in cortex and striatum (Wustmann et al., 1990[86]; Stancheva and Alova, 1994[74]). In one study, piracetam abolished the amnestic effect of 6-hydroxydopamine and restored to control values the noradrenaline level in the frontal cortex and hippocampus (Gouliaev and Senning, 1994[24]). Donepezil administration was associated with a significantly increased release of dopamine (Liang and Tang, 2006[38]), noradrenaline (Shearman et al., 2006[65]) in cortex or hippocampus, but decreased extracellular serotonin levels (Shearman et al., 2006[65]).
The effect of cannabis on brain oxidative stress, an imbalance between free radicals generation and antioxidant defense mechanisms is important in view of the evidence linking cellular damage arising from increased oxidative stress to neuronal degeneration and decline in cognitive function associated with normal aging or caused by different pathological states (Dröge, 2003[14]; Zhou et al., 2008[89]; Halliwell, 2006[26]). The present study suggests that cannabis extract alters the oxidative balance in the brain. This however, appears to be in favor of reducing lipid peroxidation. Malondialdehyde an index of lipid peroxidation activity (Gutteridge, 1995[25]), is decreased and there was significant increase of GSH, an important antioxidant defense system, especially with the highest dose of cannabis examined. Nitric oxide in brain is also decreased by cannabis administration. The significance of this finding is yet to be determined. Nitric oxide is an important signaling molecule involved in neurotransmission and in maintaining vascular tone via its vasodilator properties. Nitric oxide can be also detrimental to neural tissue if generated in excess by inflammatory cytokines due to the action of inducible nitric oxide synthase (Moncada et al., 1991[41]). In mice treated with the anticholinergic drug scopolamine so as to produce memory deficits, lipid peroxidation, though not significantly increased by scopolamine is decreased to normal value by the highest dose of cannabis. Reduced glutathione which is decreased by scopolamine is restored by cannabis administration. The extract also lessened the increase in brain nitric oxide by scopolamine. Still brain glucose decreased after cannabis administration under these conditions. These findings confirm observations in the saline-treated mice. These results are intriguing in view of the studies reporting neuroprotective effects for certain cannabinoids under experimental substances (Pazos et al., 2012[52]). These antioxidant properties of the cannabis extract can be explained by the fact that cannabis extract is not merely Δ9-THC, but rather a mixture of over 600 different chemical compounds. Cannabinoids, a group of C21 terpenophenolic compounds uniquely produced by Cannabis sativa plant are considered to be the main biologically active constituents of the Cannabis sativa plant, of which currently at least 70 are known (Brenneisen, 2006[8]). Other cannabinoids such as cannabidiol (CBD), cannabinol (CBN) and tetrahydrocannabivarin (THCV) can result in different effects from those of Δ9-THC alone (Pertwee, 2008[54]). Some cannabinoids were found to exert neuroprotective effects (Pazos et al., 2012[52]). The beneficial effects of cannabis extract on brain lipid peroxidation can also be ascribed to the flavonoids it contains. One important finding, however, was the decreased brain glucose by cannabis administration, thereby, impairing cerebral energy metabolism. This impairment of brain energetics, can explain the effect of the extract on memory function. The oxidative status of hepatic tissue was also examined. In contrast to the effect of repeated cannabis extract on oxidative stress in brain, the extract did no affect lipid peroxidation or reduced glutathione levels in the liver tissue.
The current study investigated the effect of piracetam, vinpocetine, Ginkgo biloba extract or donepezil on the memory impairment induced by cannabis extract in the water maze test. These drugs are widely prescribed to enhance memory function due to mild cognitive impairment or Alzheimer's disease (McDaniel et al., 2003[39]). Piracetam, vinpocetine, Ginkgo biloba or donepezil co-administered with cannabis resulted in significantly shorter latencies compared with mice treated with only cannabis, which indicated improved learning and memory or in other words improvement of the cognitive impairing effects of Cannabis sativa by these drugs. This effect of cannabis decreased by memory enhancing drugs with piracetam resulting in the most-shorter latency compared with the cannabis. Biochemically, cannabis altered the oxidative status of the brain with decreased MDA, increased GSH, but decreased nitric oxide and glucose. In cannabis-treated rats, the level of GSH in brain showed further increase after vinpocetine and donepezil and was markedly elevated after Ginkgo biloba. Piracetam restored the decrease in glucose and nitric oxide by cannabis. Of the memory enhancing drugs used in an attempt to counteract the effect of cannabis on memory performance, piracetam resulted in the most-shorter latency compared with the cannabis. Piracetam resulted in significantly shorter latency compared with vinpocetine over the 4 weeks of the study. The effect of piracetam was more evident during the second and third trials of the test. Moreover, only piracetam significantly increased brain glucose compared with the cannabis-treated group. The drug has been reported to reverse regional depressions in glucose metabolism in the rat hippocampus after scopolamine treatment (Piercey et al., 1987[56]). When given to patients with Alzheimer's disease, piracetam significantly improved regional glucose metabolism in most cortical areas (Heiss et al., 1988[29]). Interestingly, GSH is increased by donepezil, vinpocetine and Ginkgo biloba. In other studies, vinpocetine and piracetam increased GSH in different brain areas (Abdel-Salam et al., 2011[2]). In vitro, vinpocetine displayed scavenging activity at human therapeutic serum concentration (Horvath et al., 2002[33]). Studies indicated an antioxidant effect for Ginkgo biloba extracts. In mice, Ginkgo biloba attenuated 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced neurodegeneration of the nigrastriatal pathway, most likely due to an inhibitory effect against oxidative stress (Rojas et al., 2008[60]). In vitro, Ginkgo biloba was able to block Aβ (1-42)-induced cell apoptosis, reactive oxygen species accumulation and mitochondrial dysfunction (Shi et al., 2009[67]).
In summary, this study examined cannabis induced memory deficits in the Morris water maze, using mice. Cannabis was found to impair performance as measured by the time taken to locate a submerged platform compared to controls. The positive control scopolamine also slowed performance and added to the cannabis deficit when the two agents were tested together. The impaired memory performance is likely to involve decreased brain glucose availability as well as alterations in brain monoamine neurotransmitter levels. The cannabis induced performance deficit was attenuated by the memory enhancing drugs, with piracetam resulting in the most-shorter latency compared with the cannabis and restoring the cannabis-induced decrease in brain glucose and nitric oxide.
References
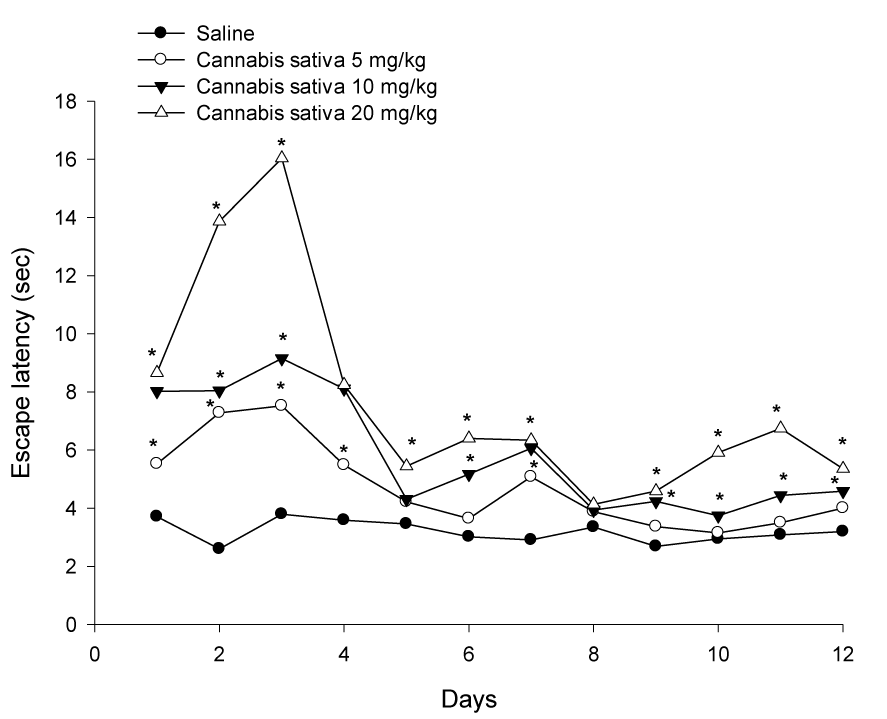
Figure 1: Effect of cannabis extract on the latency to find hidden platform in the MWM test. Cannabis was administered daily via subcutaneous route for one month and observations were done three times weekly. Asterisks indicate significant change from the saline control group.
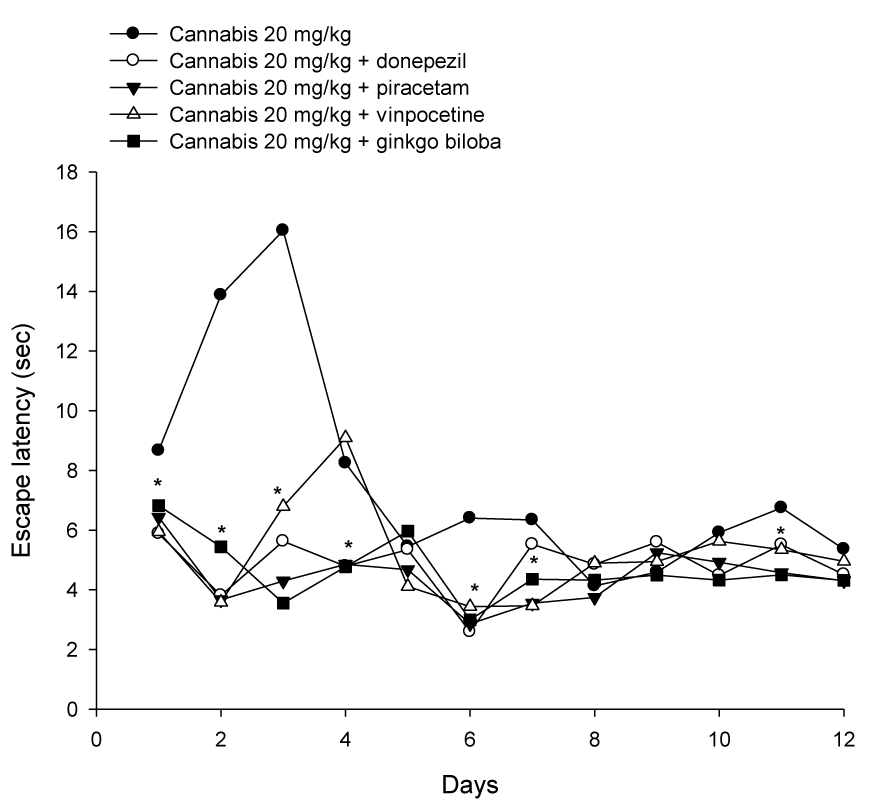
Figure 2: Effect of cannabis extract combined with donepezil, piracetam, vinpocetine or Ginkgo biloba on the latency to find hidden platform in the MWM test. Cannabis and test drugs were administered daily via subcutaneous route for one month and observations were done three times weekly. Asterisks indicate significant change from the cannabis only treated group.
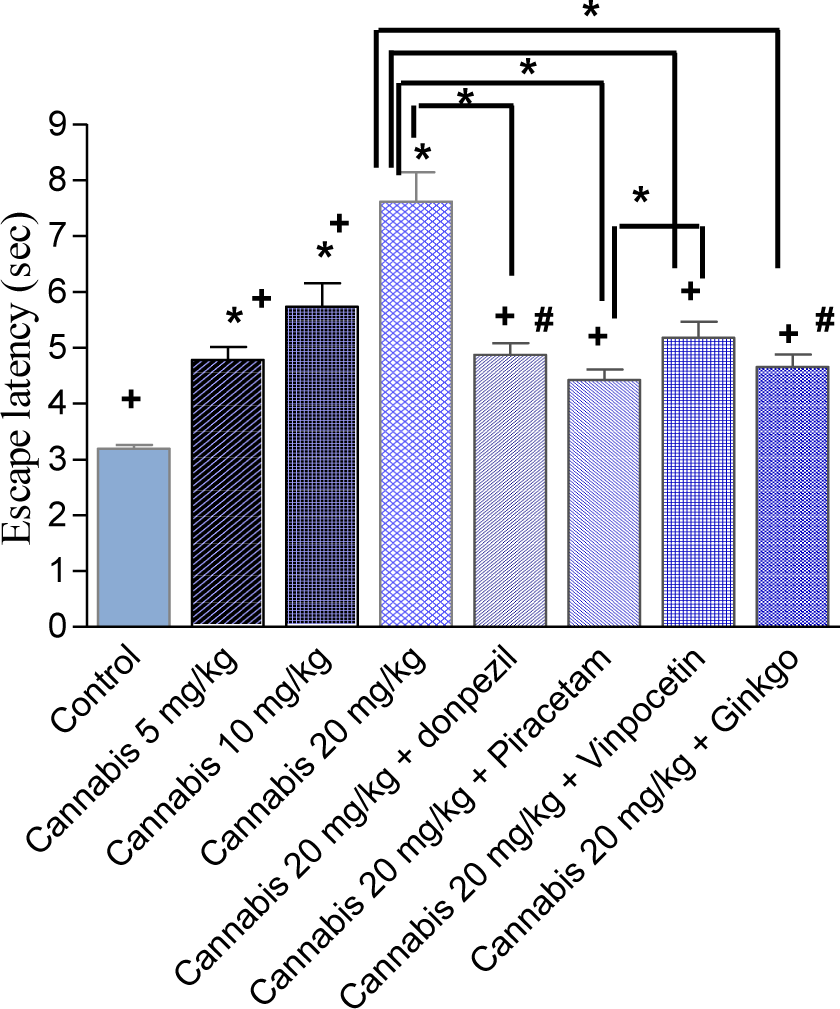
Figure 3: The average mean latency to locate a submerged platform in the MWM test over four weeks. Mice received daily injections of saline or cannabis extract (5, 10 or 20 mg/kg) alone or combined with donepezil, piracetam, vinpocetine or Ginkgo biloba and were tested three times weekly. Asterisks indicate significant change from the saline control group and between different groups as shown in the figure. The plus sign indicates significant change from the cannabis 20 mg/kg group. The # sign indicates significant change from the cannabis 10 mg/kg group. Other statistical comparisons between different treated groups are also shown and are indicated by asterisks.
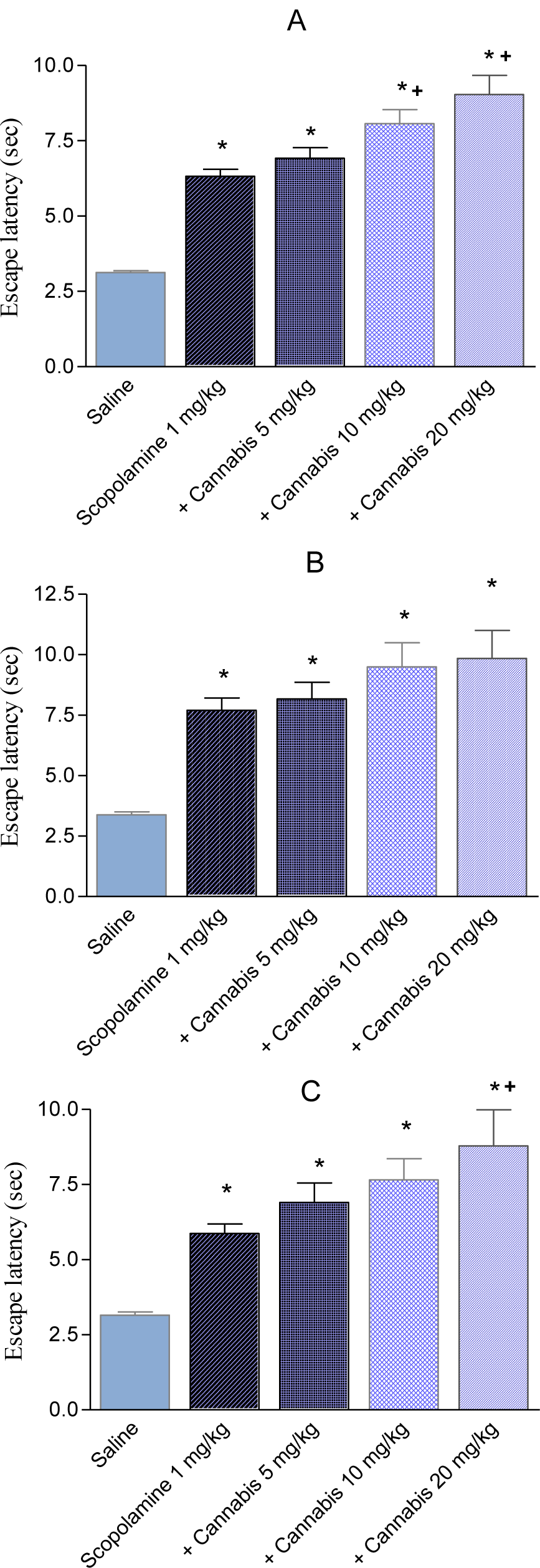
Figure 4: The average mean latency of first (A); second (B) and third (C) trial to locate a submerged platform in the MWM test over four weeks. Mice received daily injections of saline, scopolamine (1 mg/kg, s.c.) or scopolamine plus cannabis (5, 10 or 20 mg/kg) and were tested three times weekly. Asterisks indicate significant change from the saline control group. The plus sign indicates significant change from the scopolamine only or scopolamine + cannabis 5 mg/kg group.
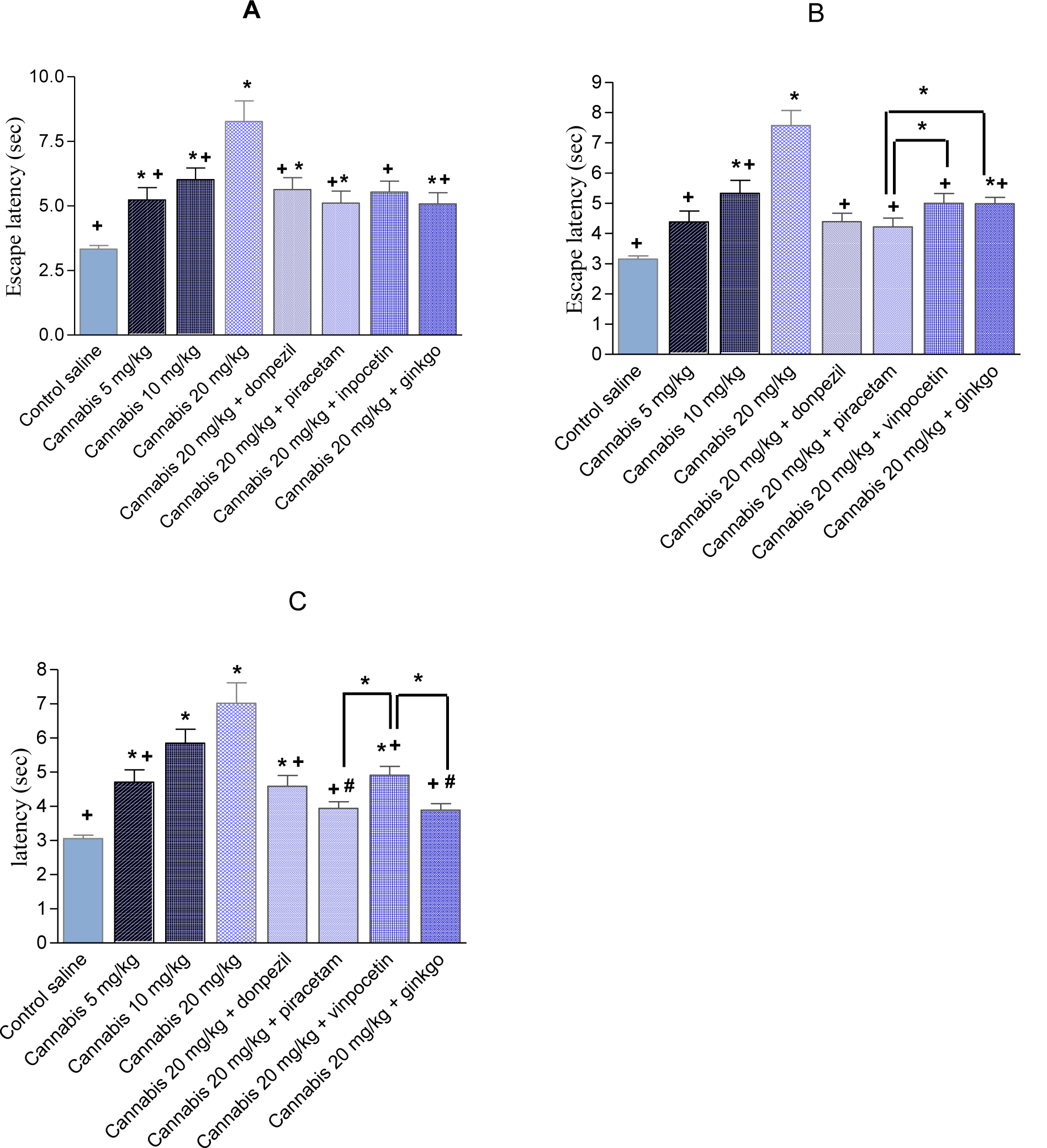
Figure 5: The average mean latency (in seconds) ± SEM of first (A); second (B) and third (C) trial to locate a submerged platform in the MWM test over four weeks. Mice received daily injections of saline or cannabis extract (5, 10 or 20 mg/kg) alone or combined with donepezil, piracetam, vinpocetine or Ginkgo biloba and were tested three times weekly. Asterisks indicate significant change from the saline control group. The plus sign indicates significant change from the cannabis 20 mg/ kg group. The # sign indicates significant change from the cannabis 10 mg/ kg group.
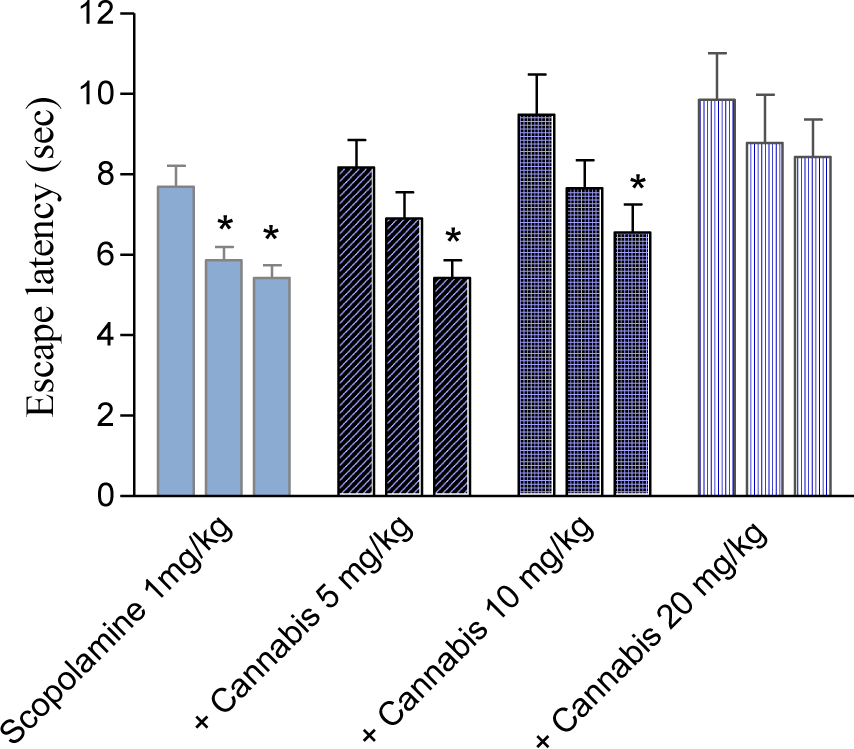
Figure 6: Effect of cannabis extract on the latency to find hidden platform in the MWM test in mice treated with scopolamine (1 mg/kg, s.c.). The columns represent the first, second and third trail, respectively for each treatment group. Asterisks indicate significant change from trial 1.
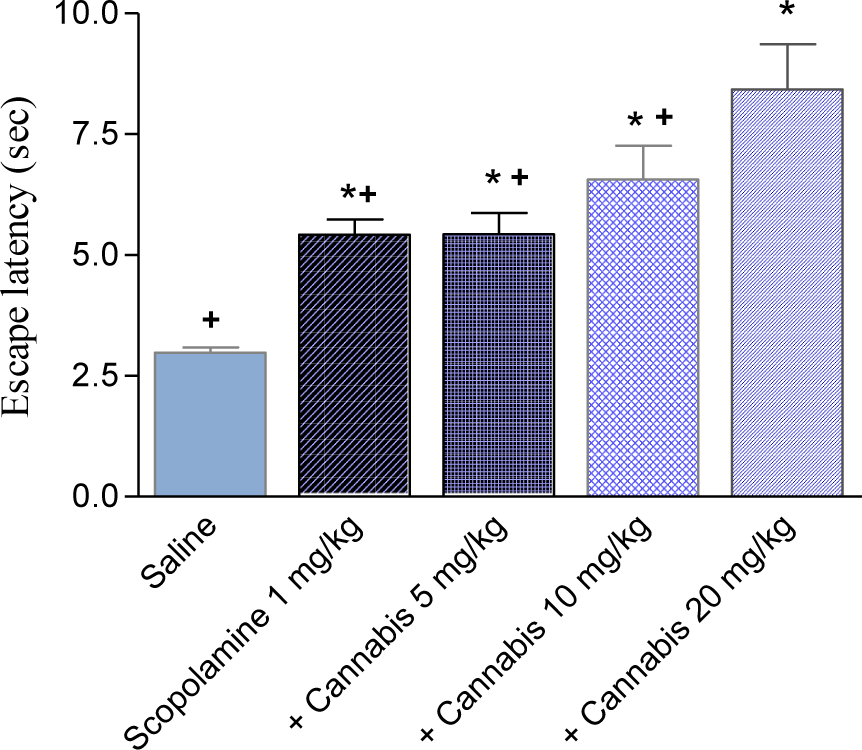
Figure 7: The average mean latency to locate a submerged platform in the MWM test over four weeks. Mice received daily injections of scopolamine (1 mg/kg, s.c.) or cannabis extract (5, 10 or 20 mg/kg) and were tested three times weekly. Asterisks indicate significant change from the saline control group. The plus sign indicates significant change from the scopolamine + cannabis 20 mg/kg group.
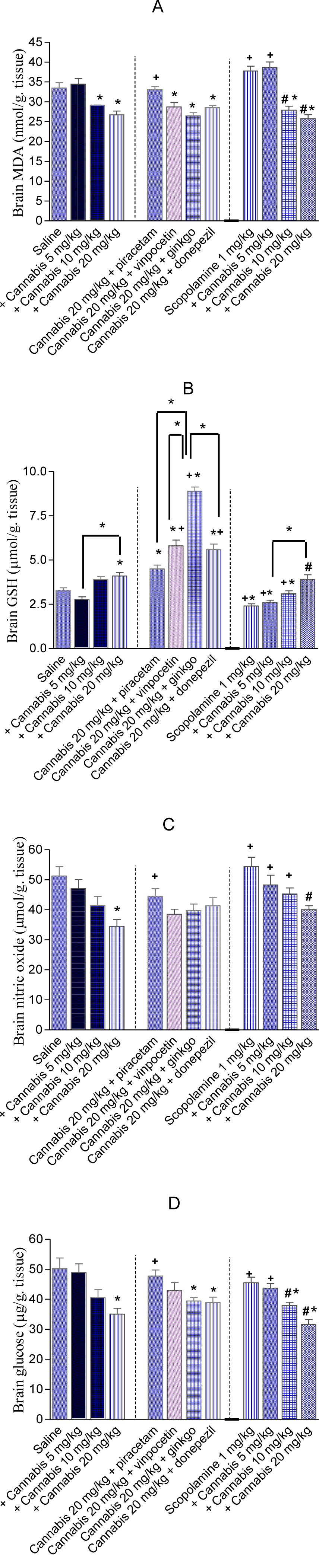
Figure 8: Effect of cannabis extract alone or in combination with piracetam, vinpocetine, Ginkgo biloba, donepezil or scopolamine on brain malondialdehyde (MDA), reduced glutathione (GSH), nitric oxide and glucose in mice. *: p< 0.05 vs corresponding saline control value. +: p< 0.05 vs. cannabis (20 mg/kg) only-treated group. #: p< 0.05 vs scopolamine only-treated group
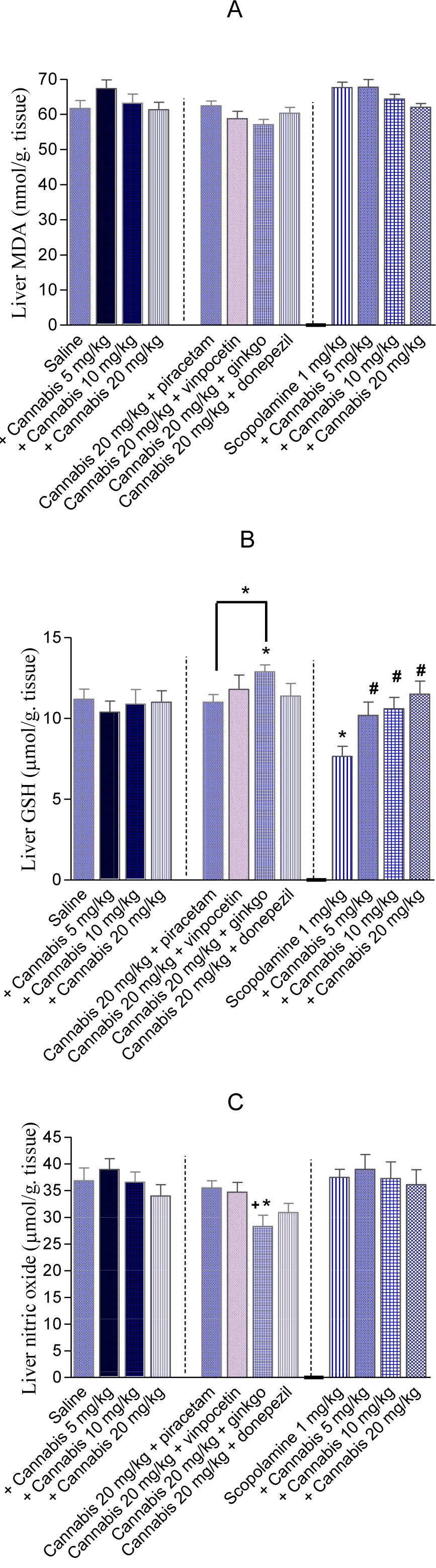
Figure 9: Effects of cannabis extract alone, cannabis + memory enhancing drugs or cannabis + scopolamine on liver malondialdehyde (MDA), reduced glutathione (GSH) and nitric oxide in mice. *: p< 0.05 vs corresponding vehicle control value. +: p< 0.05 vs. cannabis (20 mg/kg) only-treated group
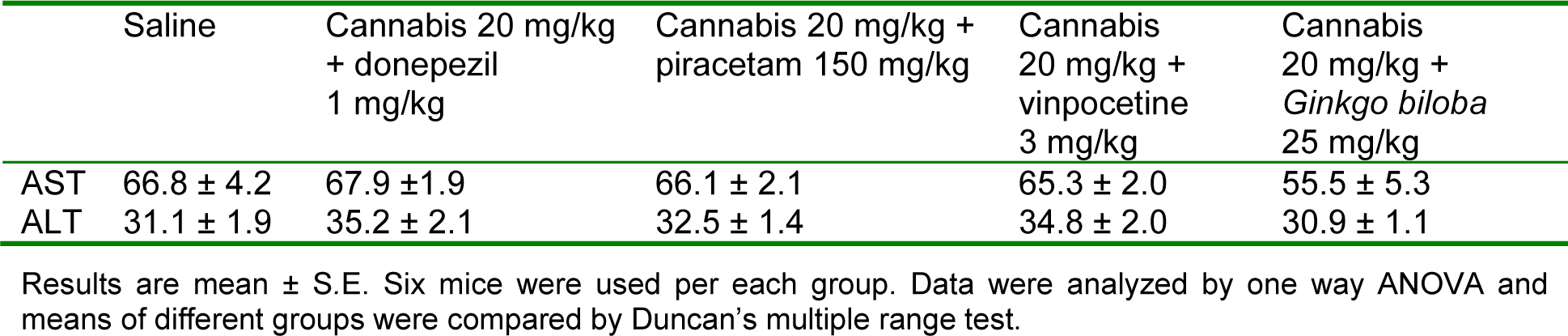
Table 2: Effect of cannabis extract given with donepezil, piracetam, vinpocetine or Ginkgo biloba on liver alanine aminotransferase (ALT) and aspartate aminotransferase (AST)
[*] Corresponding Author:
Omar M.E. Abdel-Salam, Department of Toxicology and Narcotics, National Research Centre, Tahrir St., Dokki, Cairo, Egypt, FAX: 202-33370931, eMail: omasalam@hotmail.com