Research article
Natural porous and nano fiber chitin structure from Gammarus argaeus (Gammaridae Crustacea)
Murat Kaya1[*],2, Kabil Özcan Tozak2,3, Talat Baran2,3, Göksal Sezen4, Idris Sargin2,3
1Aksaray University, Faculty of Science and Letters, Department of Biotechnology and Molecular Biology, 68100, Aksaray, Turkey2Aksaray University, Science and Technology Application and Research Center, 68100, Aksaray, Turkey
3Aksaray University, Faculty of Science and Letters, Department of Chemistry, Aksaray, Turkey
4Harran University, Faculty of Science-Literature, Department of Biology, Sanliurfa, Turkey
EXCLI J 2013;12:Doc503
Abstract
Chitin and its derivatives are commercially important biopolymers due to their applications in medicine, agriculture, water treatment, cosmetics and various biotechnological areas. Since chitin and its derivatives exhibit different chemical and physical properties depending on the source and isolation method, there is a growing demand for new chitin sources other than crab and shrimp worldwide. In this study Gammarus, a Crustacea, was investigated as a novel chitin source. Gammarus, which belongs to the family Gammaridae Crustacea, lives in the bottom of aquatic ecosystems. More than 200 species are known worldwide. One of these species, G. argaeus was investigated for chitin isolation. The alpha chitin isolated from G. argaeus was characterized by using analysis techniques such as infrared spectroscopy (FTIR), thermogravimetric analysis (TGA), X-ray diffraction (XRD) and scanning electron microscopy (SEM). All these analyses confirmed that the isolated chitin from G. argaeus was in the alpha form. Furthermore, we described that dry weight of this species contained 11-12 % chitin. SEM examination of the isolated α-chitin revealed that it was composed of nanofibrils (15-55 nm) and pores (about 150 nm).
Keywords: alpha-chitin, porosity, fibrous, natural product, characterization
Introduction
Chitin is a natural polysaccharide similar to cellulose and found in Crustacea, Insecta, Anthozoa and fungi. Chitin and chitin-derived products have great economic value and offer a wide range of potential applications in medicine, agriculture, cosmetics and waste water treatment (Felse and Panda, 1999[5]; Synowiecki and Al-Khateeb, 2003[27]; Park and Kim, 2010[20]). Chitinous products exhibit peculiar properties such as biodegradability, biocompatibility and nontoxicity. Chitin and especially its deacetylated form, chitosan, are functional polysaccharides and therefore are actively being investigated and applied. New applications in various fields are being reported for these natural products (Zang et al., 2000[31]; Liu et al., 2012[15]).
Although Crustacea has more than 50,000 defined species in aquatic ecosystems, only a few crustaceans (crab, shrimp and krill) are used in commercial production of chitin and its derivatives (Tajik et al., 2008[28]). Recently, various living organisms have been studied to meet the growing demand for chitin with different physical properties (Marguerite, 2006[16]).
Gammarus (Gammaridae: Amphipoda) is a benthic invertebrate that belongs to Crustacea class living in freshwater, brackish and saline waters (Costa and Costa, 2000[4]; Kelly et al., 2002[11]). According to Vainola et al. (2008[29]), even though there are more than 200 species of Gammarus, there are only two studies on the chitin content of this species (Muzzarelli, 1977[18]; Ivashchenko, 2002[7]). In one of these studies, chitin and chitosan were obtained from Gammarus and a water-soluble form of chitosan was prepared, and its dry weight was established to contain 8.7 % chitin (Ivashchenko, 2002[7]). In the other study, its chitin content was found to be 7 % of its dry weight (Muzzarelli, 1977[18]).
In this present work, the isolation of chitin from G. argaeus, a Gammarus species, known to inhabit Turkey and physicochemical structure of the extracted chitin are detailed. The percentage of chitin content of the G. argaeus was established and the characterization of the isolated chitin was carried out with FTIR, TGA, XRD, SEM techniques.
Materials and Methods
Collection of samples
Samples were sieved with a 500 micron sieve after excavating a 0.5 m² muddy structure at the bottom of Soysalli Lake (Central Anatolia, Turkey) on 02.01.2013. Adult G. argaeus individuals left in the sieved portion were collected in plastic container filled with 500 ml distilled water and then taken to the laboratory. Samples brought to the laboratory were rinsed repeatedly with distilled water on a 500 micron sieve; this way, particles already stuck on Gammarus individuals were removed. Then, samples were dried on paper at room temperature to constant weight. It was estimated that 20 g of Gammarus in dry weight was harvested from an area of 0.5 m². It was noted in a previous study that G. argaeus inhabited in Soysalli Lake (Özbek, 2010[19]).
Chitin extraction
The demineralization process was carried out to remove the inorganic content as follows: 2 g of washed and dried Gammarus was ground to fine powder. Then it was dried in oven for 2 h at 60 ºC. One gram of sample dried in oven was refluxed with 100 ml of 1 M HCI solution for 2 h at 55-65 ºC. Later on, the sample was filtered off and the residue was rinsed thoroughly with deionized water. To achieve deproteinization step, the filtrate was treated with 100 ml of 1M NaOH solution for 23 h at 75-80 ºC. The mixture was filtered off and washed repeatedly with deionized water. The sample was then placed in a vacuum oven at 70 ºC. Following the drying process, it was incubated in an organic solution mixture containing chloroform, methanol, and water (in the ratio of 1:2:4) for an hour for elimination of lipids, decolourisation and bleaching.
Fourier transform infrared spectroscopy (FTIR)
The IR spectra of extracted chitin from G. argaeus were measured using a Perkin Elmer FTIR Spectrometer over the frequency range of 4000-625 cm-1.
Thermogravimetric analysis (TGA)
EXSTAR S11 7300 was used to obtain TG and DTG curves at the thermal degradation of chitin at a heating rate of 10 ºC min-1.
X-ray diffraction (XRD)
X-ray diffraction spectra were obtained at 40 kV, 30 mA and 2θ with the scan angle from 5º to 45º using a Rigaku D max 2000 system (Harran University, HÜMEL).
Scanning electron microscopy (SEM)
The surface morphology of chitin extracted from G. argaeus was analysed by using EVO LS 10 ZEISS scanning electron microscope. The sample was coated with gold for SEM analysis by Sputter Coater (Cressingto Auto 108).
Results and Discussion
Chitin content
Chitin is the second most abundant structural biomolecule present in the biosphere. It forms the exoskeletons of organisms such as Arthropoda, Anthozoa and is also found in a multitude of organisms from bacteria and fungi (Muzzarelli, 1977[18]; Rinaudo, 2006[23]). Although many species in nature possess chitin structure, chitin structure of very few organisms has been analyzed in detail so far. In the current study, chitin content of G. argaeus, a Cructacea species, was investigated and established to contain 11-12 % chitin of dry weight. In previous studies, it was found that dry Gammarus contained 7 % and 8.7 % chitin (Ivashchenko, 2002[7]; Muzzarelli, 1977[18]). Gammarus densely populates the bottom mud of shallow lakes and disintegrates plant wastes and assumes a role in energy transmission (Christie and Kraufvelin, 2003[3]). In this study, 20 g of Gammarus on dry basis was obtained from 0.5 m2 area at the bottom of Soysalli Lake, shallow water. The area of the lake is approximately 55,000 m2. The lake is estimated to house about 2,200 kg Gammarus at dry weight. This demonstrates that about 242-264 kg alpha chitin in pure fibre structure can be obtained through processing Gammarus in the lake.
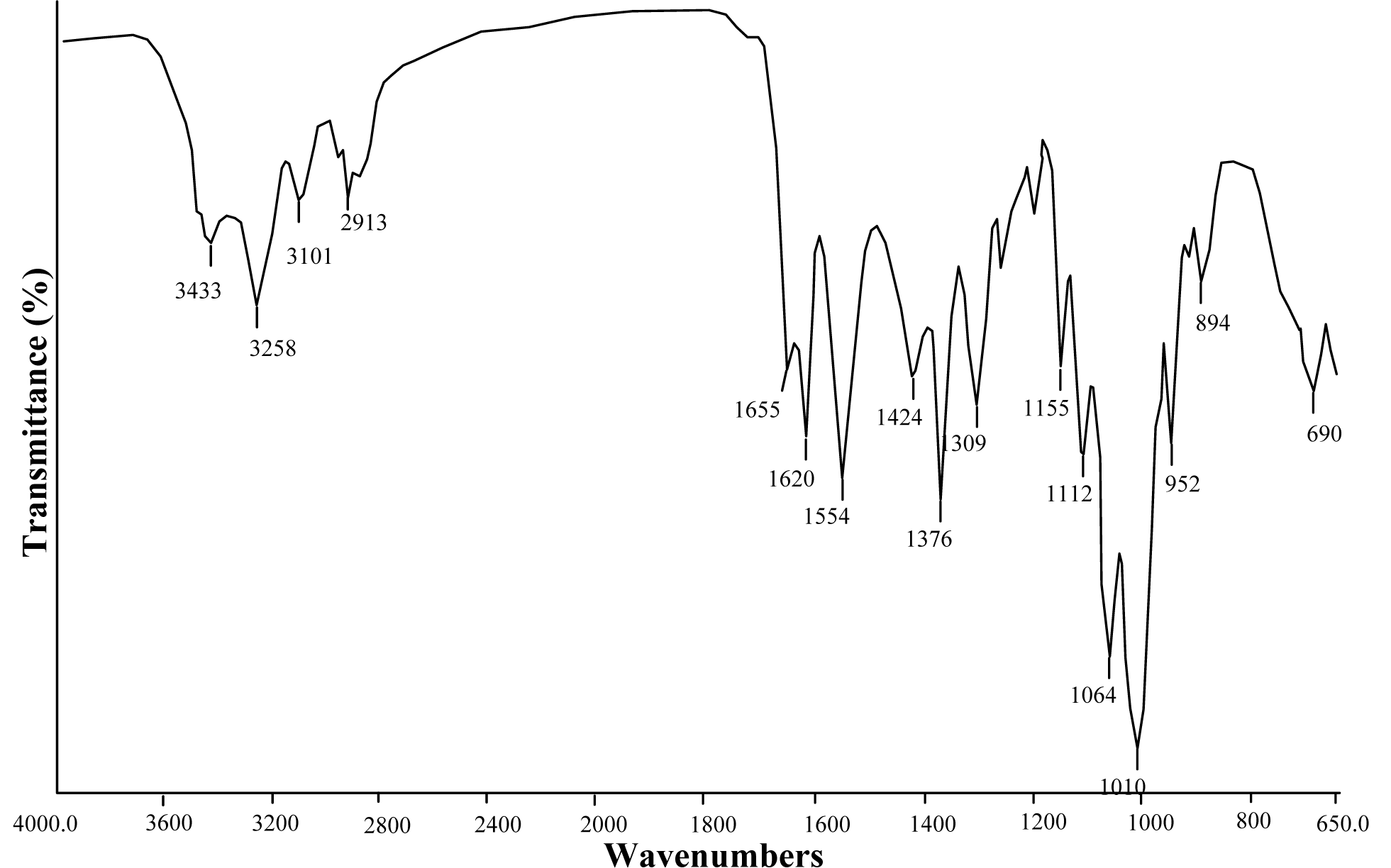
FTIR
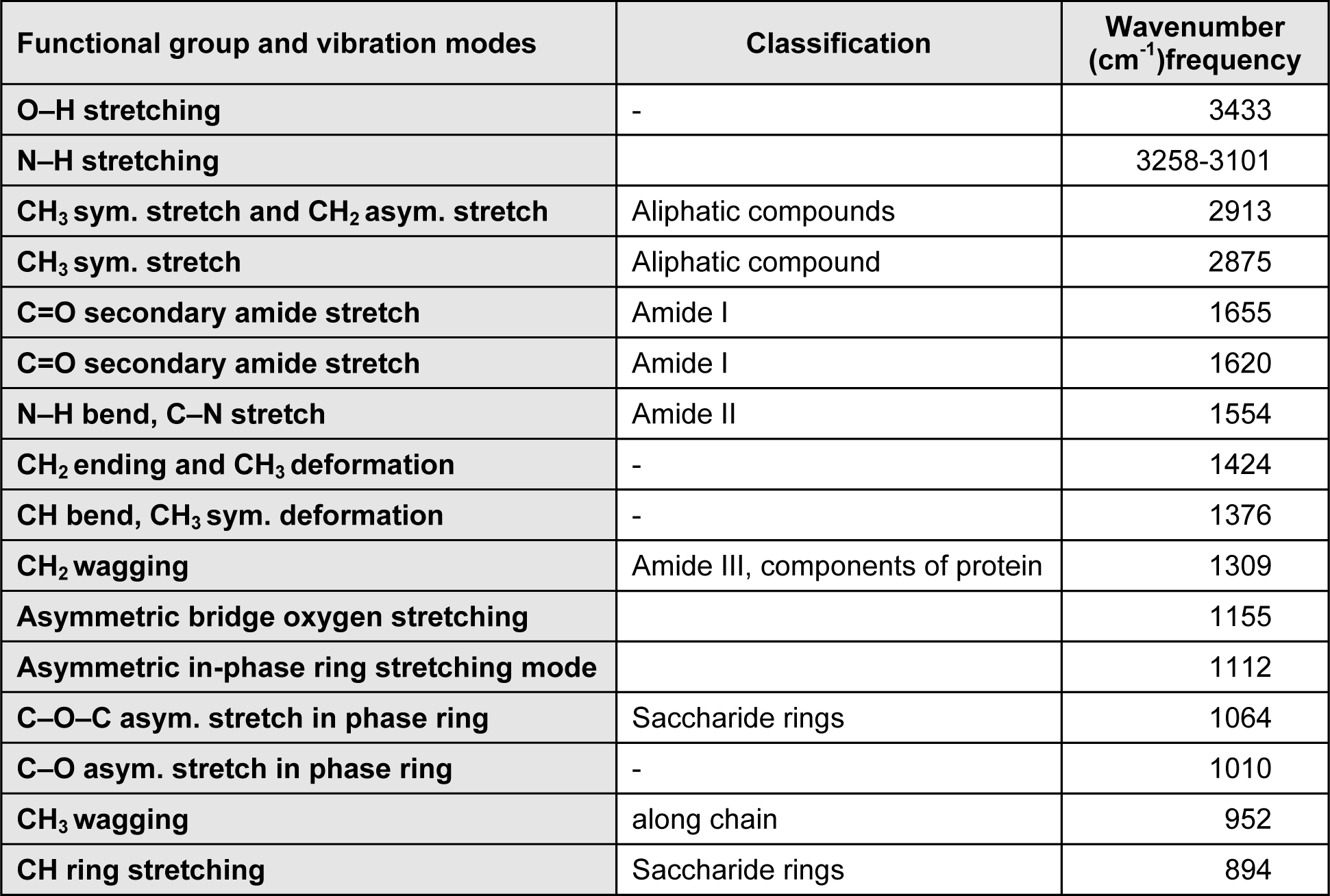
IR spectrum analysis is an important method in chitin characterization. Chitin is found in nature in 3 different forms; alpha, beta and gamma (Rudall, 1973[24]; Cabib et al., 1988[2]). In IR spectrum of alpha chitin, 3 peaks at about 1650, 1620 and 1550 cm-1 indicate that chitin examined is in alpha form (Focher et al., 1992[6]; Rinaudo, 2006[23]; Lavall et al., 2007[14]). Unlike alpha form, in beta form, a single peak is observed at about 1650 cm-1 (Kurita et al., 1993[13]; Lavall et al., 2007[14]; Rinaudo, 2006[23]). As seen in Figure 1(Fig. 1), two separate peaks were observed at 1650 and 1620 cm-1 in the IR spectrum of chitin obtained from G. argaeus. This shows us that the chitin structure of G. argaeus is in alpha form. Alpha form of chitin is quite common and it can be found in numerous studies (Minke and Blackwell, 1978[17]). Beta form was observed in squid pens (Al Sagheer et al., 2009[1]; Rudall, 1969[24]), and gamma form was established to be found in fungi and yeast (Al Sagheer et al., 2009[1]). Also, gamma chitin is known to be present in the insect cocoons (Kenchington, 1976[12]). Other peaks observed in IR spectrum of the alpha chitin extracted are given in Table 1(Tab. 1).
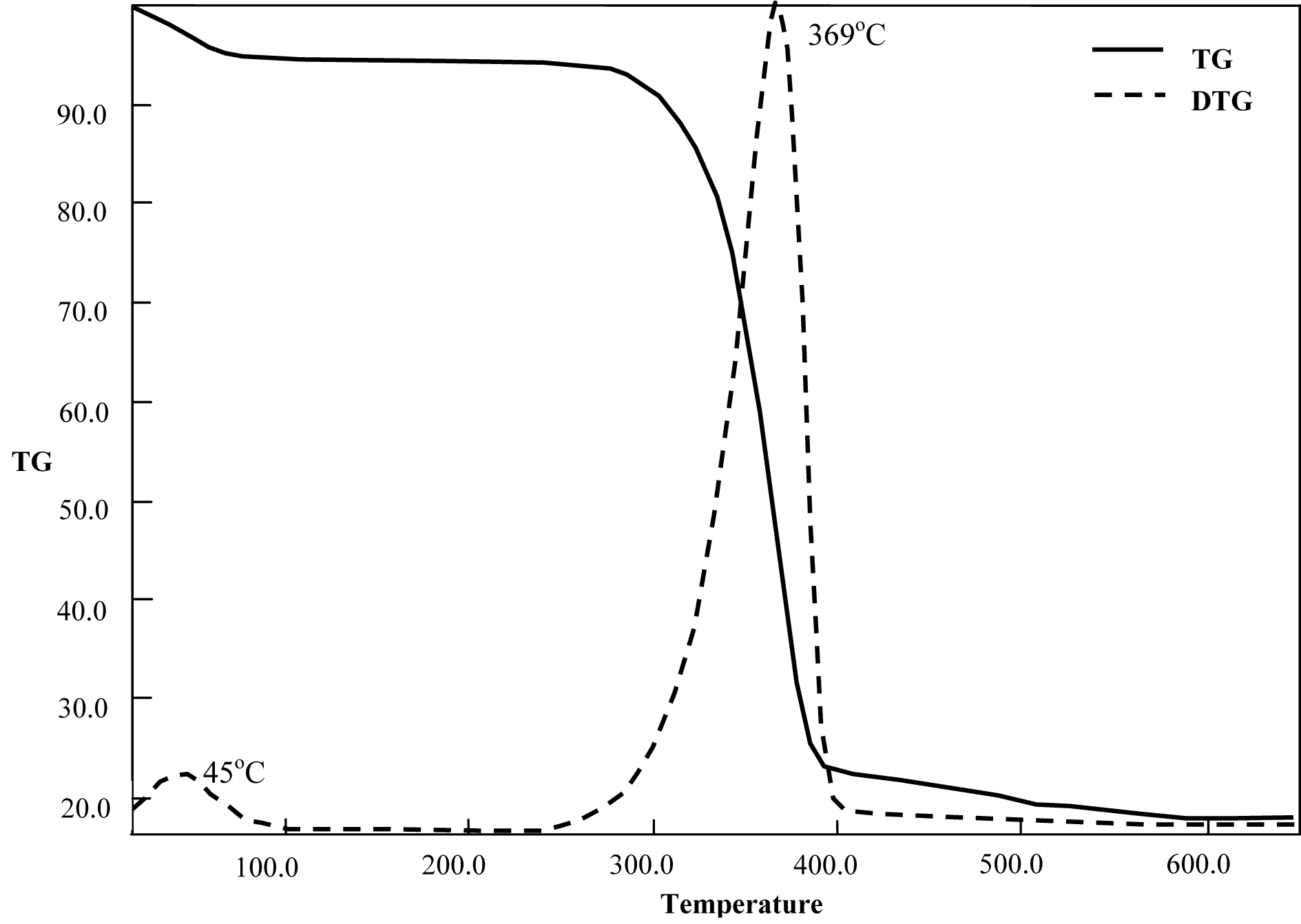
TGA
In the thermogram of chitin extracted from G. argaeus, mass loss was observed in two steps (Figure 2(Fig. 2)). The mass loss in the first stage can be attributed to the evaporation of water in the structure and the loss in second stage was due to the decomposition of polysaccharides in structure. In previous TGA and DTG studies conducted on chitin, mass losses in 2 steps similar to the current study were reported (Jayakumar et al., 2009[9]).
In a previous study, decomposition of chitin structure with TGA at about 350-380 ºC indicated the alpha form of extracted chitin, while the one at about 300 ºC showed the gamma form of the chitin (Jang et al., 2004[8]). In this study, TGA and DTG examinations showed that 74 % of the chitin structure decomposed at around 369 ºC, and chitin obtained from G. argaeus was established to be in the alpha form.
In a previous study, decomposition of chitin structure with TGA at about 350-380 ºC indicated the alpha form of extracted chitin, while the one at about 300 ºC showed the gamma form of the chitin (Jang et al., 2004[8]). In this study, TGA and DTG examinations showed that 74 % of the chitin structure decomposed at around 369 ºC, and chitin obtained from G. argaeus was established to be in the alpha form.
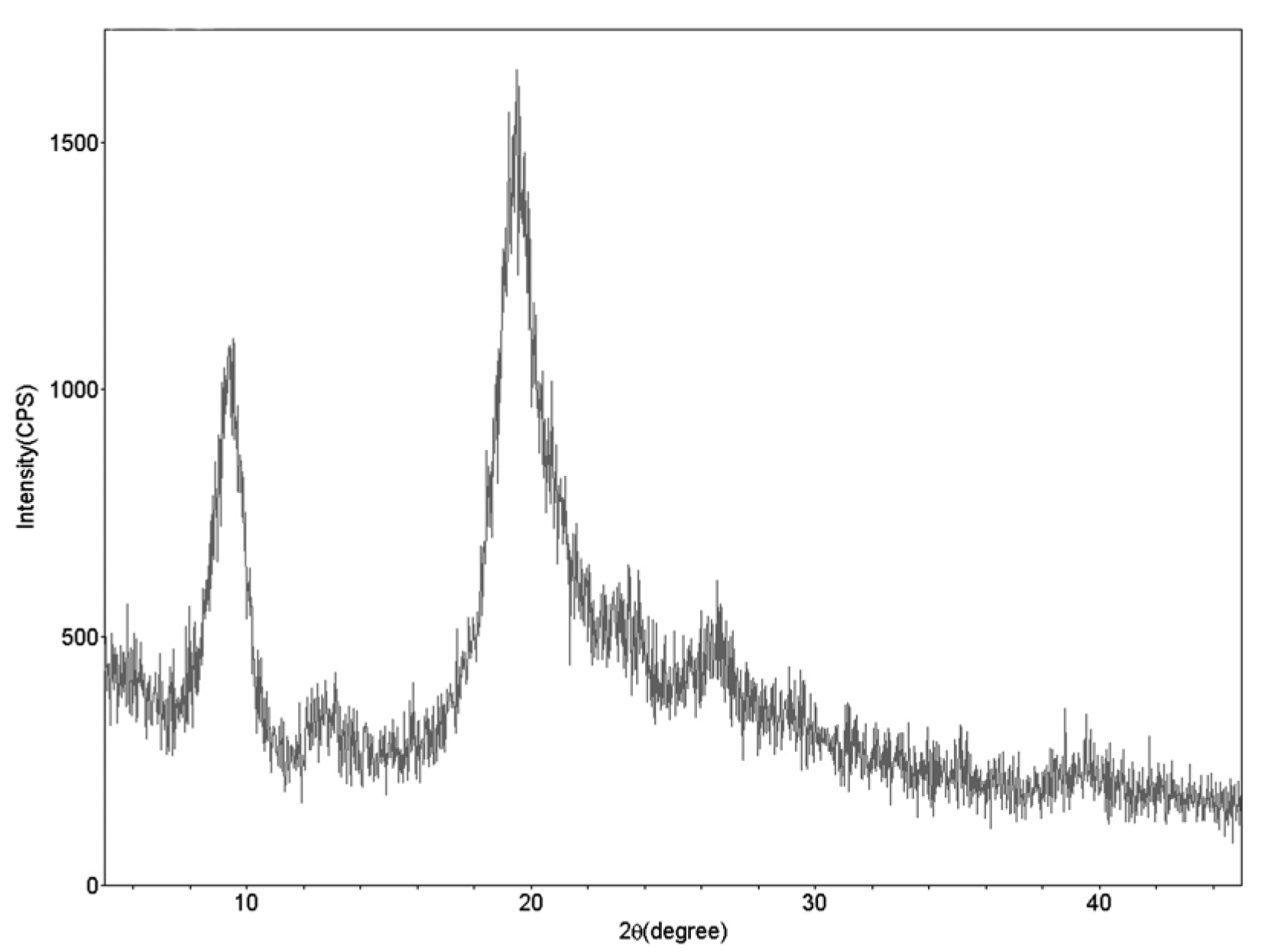
XRD
In X-ray diffraction spectrum of chitin from G. argaeus, two strong (9.24 and 19.44º) and 3 weak (12.8, 23.4 and 26.58º) peaks were observed (Figure 3(Fig. 3)). In previous XRD studies conducted on alpha chitin from various organisms, two strong peaks at 9 and 19º, and weak peaks at 12, 21, 23, and 26º were observed in the spectra (Yen et al., 2009[30]; Sajomsang and Gonil, 2010[26]; Juárez-de La Rosa et al., 2012[10]; Liu et al., 2012[15]). XRD examination in this study revealed crystalline structure of alpha chitin polymer found in G. argaeus.
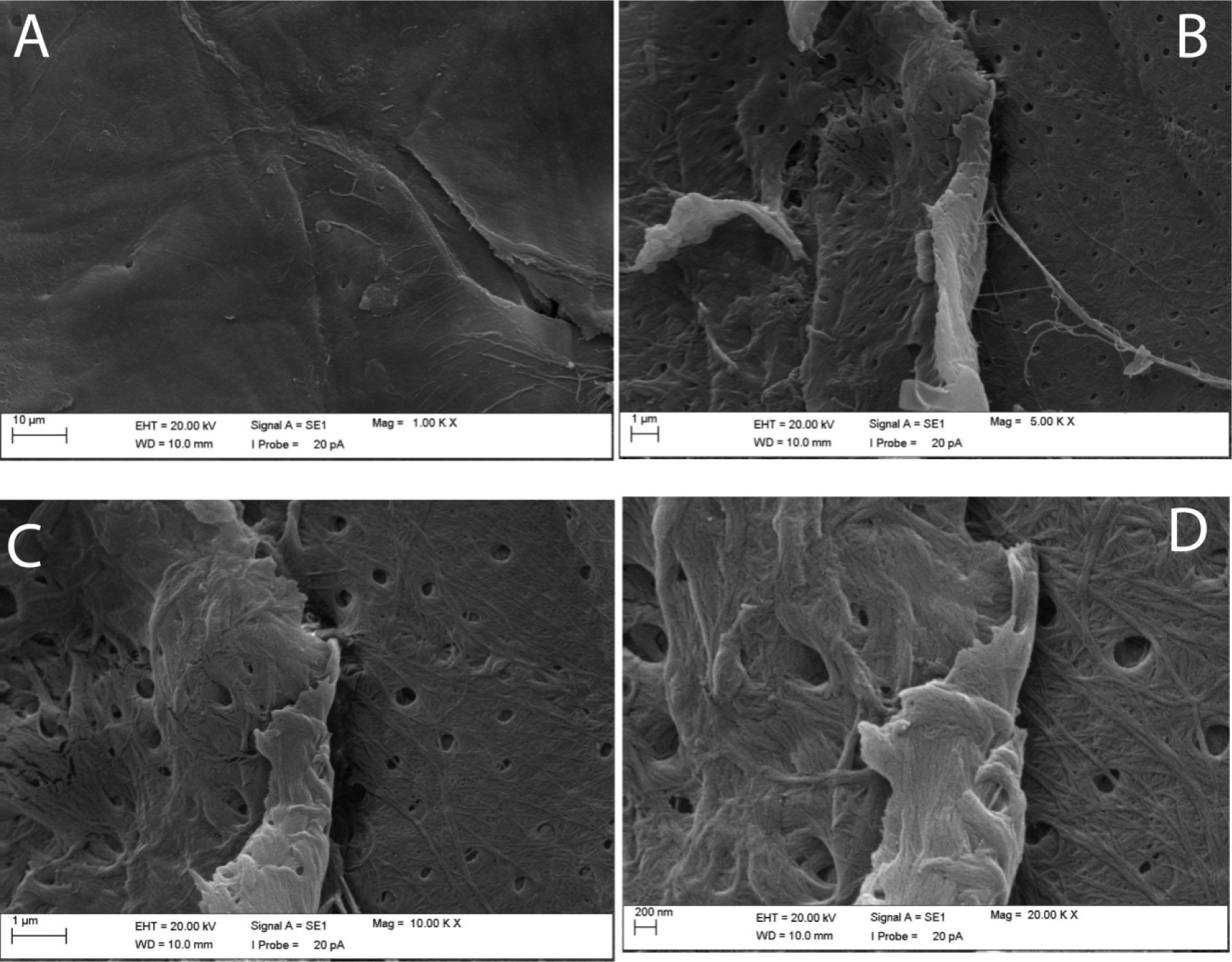
SEM
Surface of chitin obtained from G. argaeus was found to have a smooth quality at 1000x zoom (Figure 4A(Fig. 4)), but it was observed to be fibrous with increase in zoom and especially at 20000X magnification (Figure 4D(Fig. 4)). Also zooming at 5000, 10000, and 20000X magnification revealed the pores of 150 nm size on chitin surface (Figures 4B, C and D(Fig. 4)). Width of fibrils was observed to be in the range of 15 to 55 nm.
Conclusion
With this study, it was established that the dry weight of G. argaeus species contained 11-12 % chitin. The structure was found to be α-chitin as a result of FTIR, TGA and XRD analyses. The SEM analysis showed that the obtained chitin had a fibrous structure with sizes ranging between 15 and 55 nm and porous structure with about 150 nm sizes. Nano fibre polymeric structures are generally used in textile, membrane (filtration), controlled drug delivery, wound dressings, biosensors and nano composites for dental applications (Ravi Kumar, 2000[22]; Pillai and Sharma, 2009[21]). Characterization studies of α-chitin isolated from G. argaeus in this study can give an insight into possible applications of chitin-derived products and offers G. argaeus as an alternative chitin source.
References
[*] Corresponding Author:
Murat Kaya, Aksaray University, Faculty of Science and Letters, Department of Biotechnology and Molecular Biology, 68100, Aksaray, Turkey; Tel.: +90-382-288-2184; Fax: +90-382-288-2125, eMail: muratkaya3806@yahoo.com