Research article
Irrational antibiotic prescribing: a local issue or global concern?
Shiva Hashemi1, Azadeh Nasrollah1, Mehdi Rajabi1[*]
1Department of Clinical Pharmacy, Islamic Azad University Pharmaceutical Sciences Branch, Tehran, IranEXCLI J 2013;12:Doc384
Abstract
Resistance to antibiotics is a major public-health concern and antibiotic use is being ever more recognized as the main discriminatory pressure driving this resistance. The aim was to assess the outpatient usage of antibiotics in teaching hospitals in various parts of capital city of Iran, Tehran and its association with resistance. 600 outpatient antibiotic prescriptions between December 2011 and May 2012 were reviewed in our teaching hospitals. All prescriptions were scrutinized in order to evaluate the antibiotic prescribing. The medical doctors from all grades were asked to note the chief complaints and the most possible diagnosis on each prescription. Clinical data, patient demographic and ultimately the total quantities of antibiotics were recorded. Our data was then compared against the major antibiotic guidelines and similar studies in other countries. The most common prescribed antibiotics are Penicillins (Penicillin, Co-Amoxiclav and Amoxicillin) (40 %), Cephalosporins (Cefixime, Cephalexin and Ceftriaxone) (24.5 %) and Macrolides (particularly Azithromycin) (15.3 %). In total, 18.2 % of cases were combinational antibacterial therapies (≥ 2). The most common diagnosis was upper respiratory tract infections as common cold (29.2 %) and sore throat (11.8 %). Directions (instructions for use) of 58 % of selected antibiotics were acceptable. Parenteral administration remains the common route of administration with 22 % of all reviewed prescriptions. Based on Cochrane reviews the antibiotic prescribing was unjustified in 42.7 % of the cases. The prescribing habit, correct diagnosis and the use of antibiotics need instant consideration. These data can provide useful information for assessing public-health policy that aims to reduce the antibiotic use and resistance levels.
Keywords: antibiotic prescribing, resistance, review, outpatients
Introduction
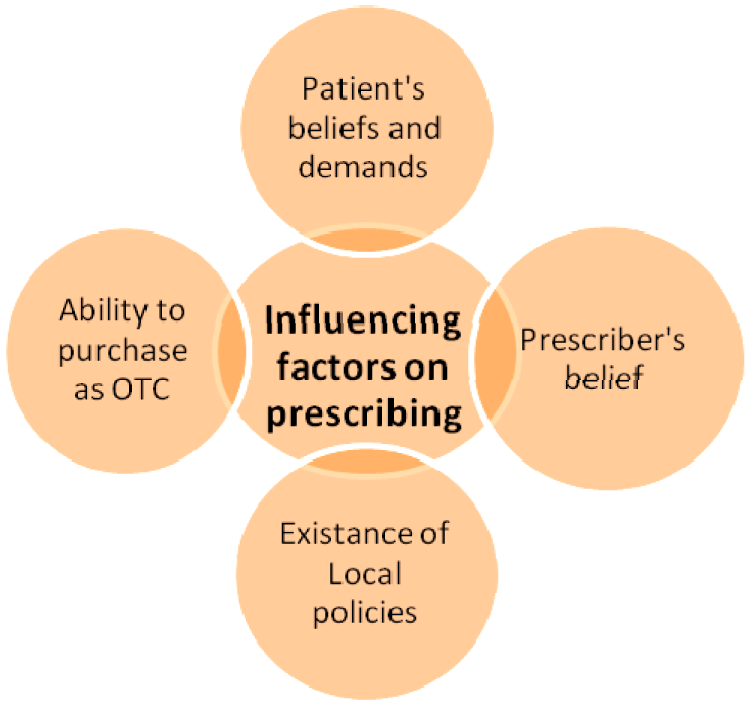
An antibiotic is an agent that inhibits bacterial growth or kills bacteria. Antibiotic is any substance produced by a microorganism that is antagonistic to the growth of other microorganisms in high dilution. According to the World Health Organization (WHO), resistance to the antibiotics is one of the biggest threats to human health today. Antibiotic resistance is defined as the resistance of microorganisms to antimicrobial agents and that happens when bacteria changes to protect itself from antibiotics. The inappropriate antibacterial treatment and the overuse of antibiotics have contributed to the emergence of antibacterial-resistant. The emergence of antimicrobial resistance (AMR) is a major concern in public health and antibiotic use is being ever more recognized as the main discriminatory pressure driving this resistance. This problem cannot be prevented but its prevalence can be decreased (Gould, 2009[7]). Unfortunately antibiotic resistance is increasing in all over the world (Baktygul et al., 2011[4]; Gould, 2009[7]; Hadi et al., 2008[8]; Sumpradit et al., 2012[21]) and according to the WHO reports, Iran (location as in Figure 1(Fig. 1)) is one of the top 20 highest medicine consumption countries. Iranians are amongst some of the highest users of antibiotics in the world. AMR results in increased morbidity, mortality, cost of health care and ultimately with time decrease in the rate of successful treatment. Several influential factors do exist when prescribing the antibiotics. Prescriber's factors such as fear, lack of information, excessive and unnecessary antibiotic prescribing, incorrect dosage or route of administration, antibiotic prescribing for non-bacterial infections, patient demands and self prescribing. However, inappropriate prescribing of antibiotics by the physicians is the most important recognized factor (Harbarth et al., 2002[9]; Vazquez-Lago et al., 2011[23]; Cadieux et al., 2007[5]; Ali et al., 2006[2]) (Figure 2(Fig. 2)). Prevention of antibiotic misuse is the key for controlling the antibiotic resistance) (Sintchenko et al., 2008[20]; Harbarth et al., 2002[9]). Approximately, 80 % of antibiotic are used in primary care and the rest in hospitals (Kotwani and Holloway, 2011[12]; Kuyvenhoven et al., 2003[14]; Ashiru-Oredope et al., 2012[3]). Therefore, it is hugely important to review the outpatient prescribing on regular intervals and have a robust system in placed to modify the areas that needs improving.
Although the “irrational prescribing” is not a new global topic and has been a reason for concern for many years, still many countries are not paying enough attention to this old but ongoing problem across the world. We were therefore, prompted to initiate this novel and unique pilot study to investigate the extent of this concern and then further our studies nationally to tackle the current problem.
In this descriptive study, 600 outpatient antibiotic prescriptions for the period of six months were reviewed in our teaching hospitals. The aim of this study was to assess the outpatient usage of antibiotics in our teaching hospitals in various parts of Tehran and its possible association with antibacterial resistance. The antimicrobial prescribing in academic health centers were compared against major guidelines, with the view of developing a controlling program on antibiotics prescribing and reduction of mentioned problems associated with irrational prescribing of antibiotics.
Definitions
These definitions are obtained from the WHO and are not necessarily the same as what is referred to in Iran due to translational error or misinterpretation of the public.
Rational prescribing: prescribe appropriate medication for the right patient at the right dose and interval for the right duration of therapy (Kumar et al., 2010[13]).
Definition of common infection:
- Common cold: it is a viral infectious disease that affects the upper respiratory tract; but the public beliefs that common cold is a bacterial infection
- Sinusitis: allergic, viral or infective inflammation of sinuses
- Sore throat: painful or irritable throat that usually is caused by acute pharyngitis
- Pharyngitis: viral, bacterial or fungal inflammation of throat, mostly the acute form caused by viral infection
- Laryngitis: inflammation of the mucous membrane of the larynx, that is caused by infective or non-infective agent
- Urinary Tract Infection (UTI): inflammatory disease in urinary tract caused by bacterial infection.
Materials and Methods
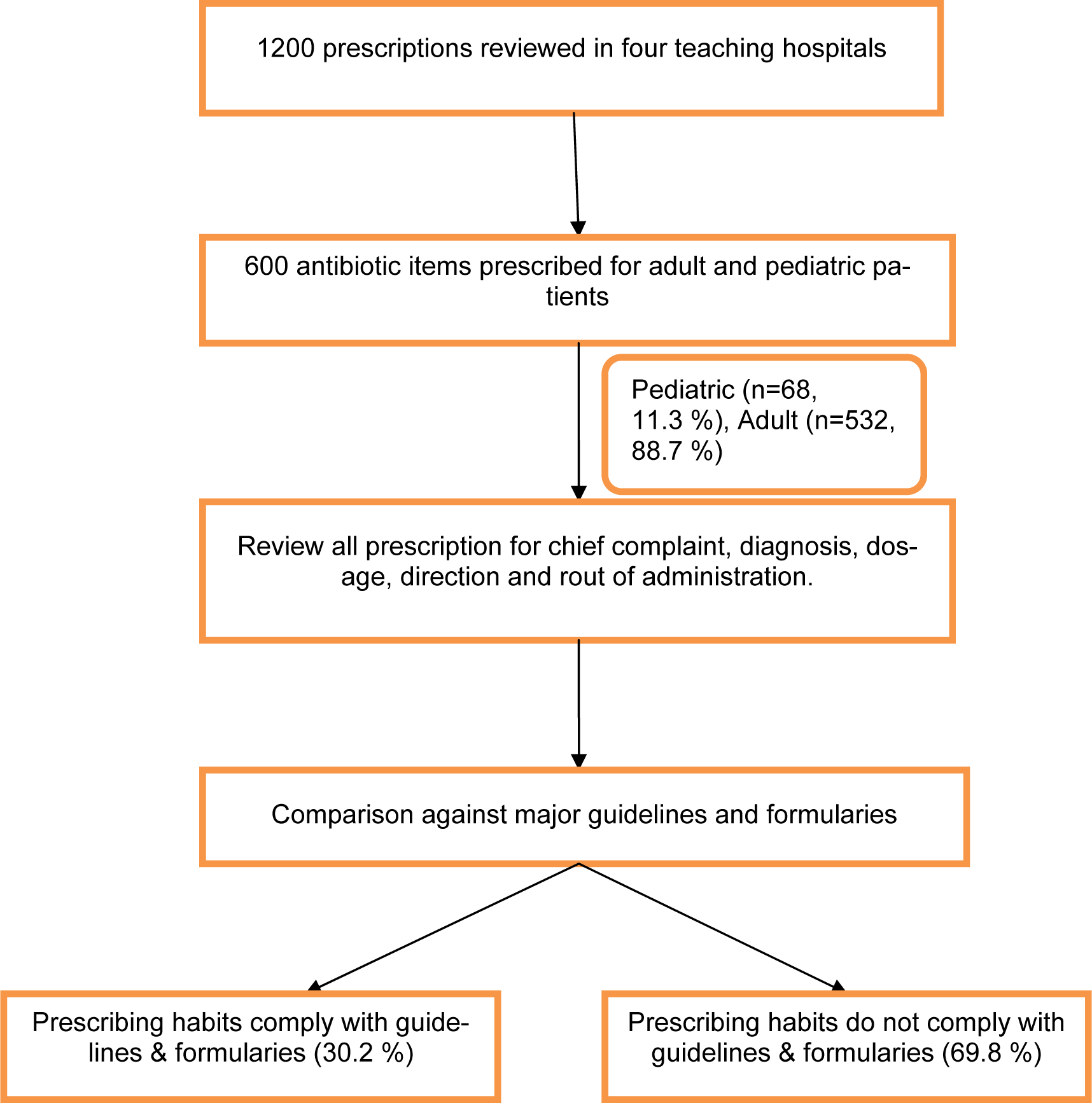
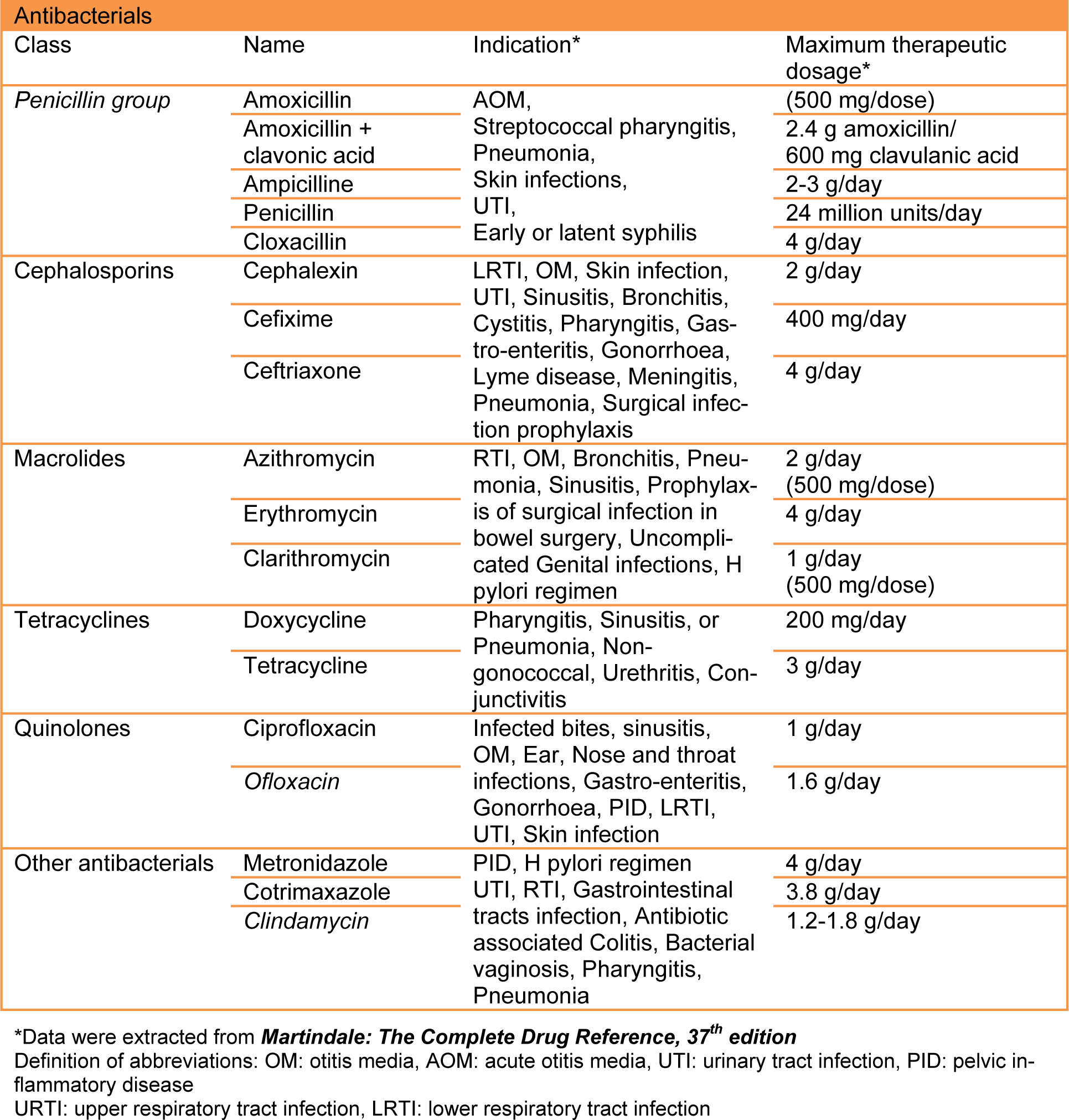
As part of novel and ongoing prescribing assessment in Iran, 600 outpatient antibiotic items (recommended numbers by WHO) (WHO, 2008[24]) were selected in random, by scrutinizing in excess of 1200 prescriptions between December 2011 and May 2012 from outpatient clinics in teaching hospitals. The Islamic Azad University Teaching Hospitals are one of the biggest medical schools in Iran, training in excess of 900 medical students per year. All graduates working in various grades and specialties covering the outpatient clinics which are located in the North (Javaheri hospital), South (Amiralmomenin hospital), East (Buali hospital) and West (Ghods) of capital city, Tehran. Due to the location of these centers, patients who are using the offered medical services in our sample size would be from different backgrounds and fulfill all demographic factors. All prescriptions were scrutinized in order to evaluate the antibiotic prescribing habits and any errors that might influence the patient's therapy. The method for classification of antibiotics was Anatomical Therapeutic Chemical (ATC). The medical doctors from all grades were asked to note the chief complaints and the most likelihood diagnosis on each prescription. In order to avoid any bias, the medical teams were not aware of the data gathering. Clinical data such as diagnosis, type of antibiotics, routes of administrations, doses, directions and ultimately the total quantities of antibiotics as well as patient's demographic data including age and sex were recorded. Antibiotics were chosen from essential medicine WHO model list and are the most commonly prescribed ones in the teaching hospitals (Table 1(Tab. 1)). In this study, patients of all ages regardless of number of prescribed antibiotics were included. Acquired data was then compared against major antibiotic guidelines and cross-referenced against Cochrane studies and Martindale. Data analysis was performed using Microsoft excel and SPSS 18 statistical program.
Results
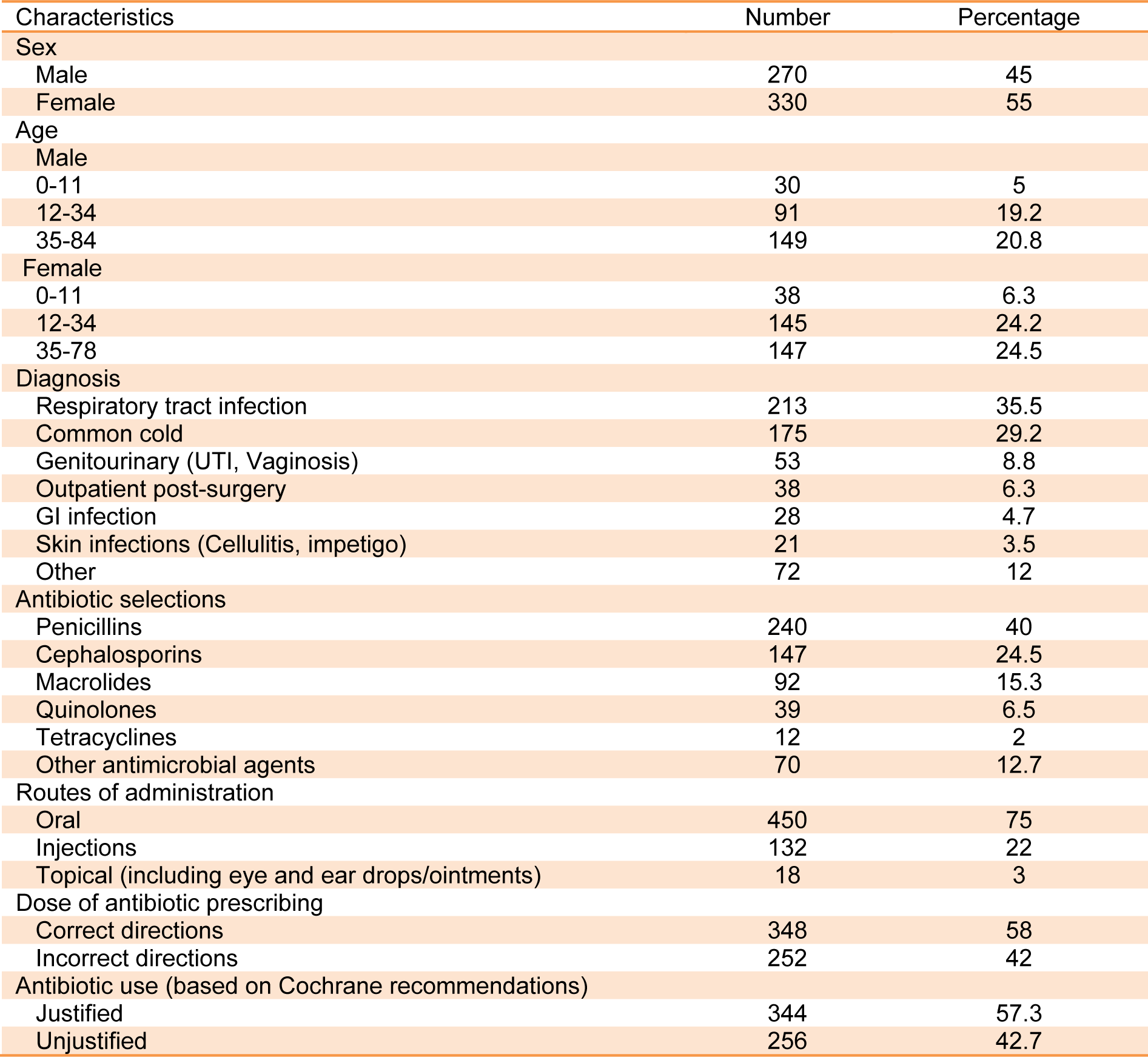
Based on essential medicines WHO model list, the most common antibiotics which are referred as efficacious, safe and cost-effective were selected. This selection is also considered on the basis of current and estimated future public health needs. Moreover, in order to get the best sample, the population in our study was based on random selection. This random sampling consists of 5 % paediatric males, 40 % adult males, 6.3 % paediatric females and 48.7 % were adult females in total (Figure 3(Fig. 3)).
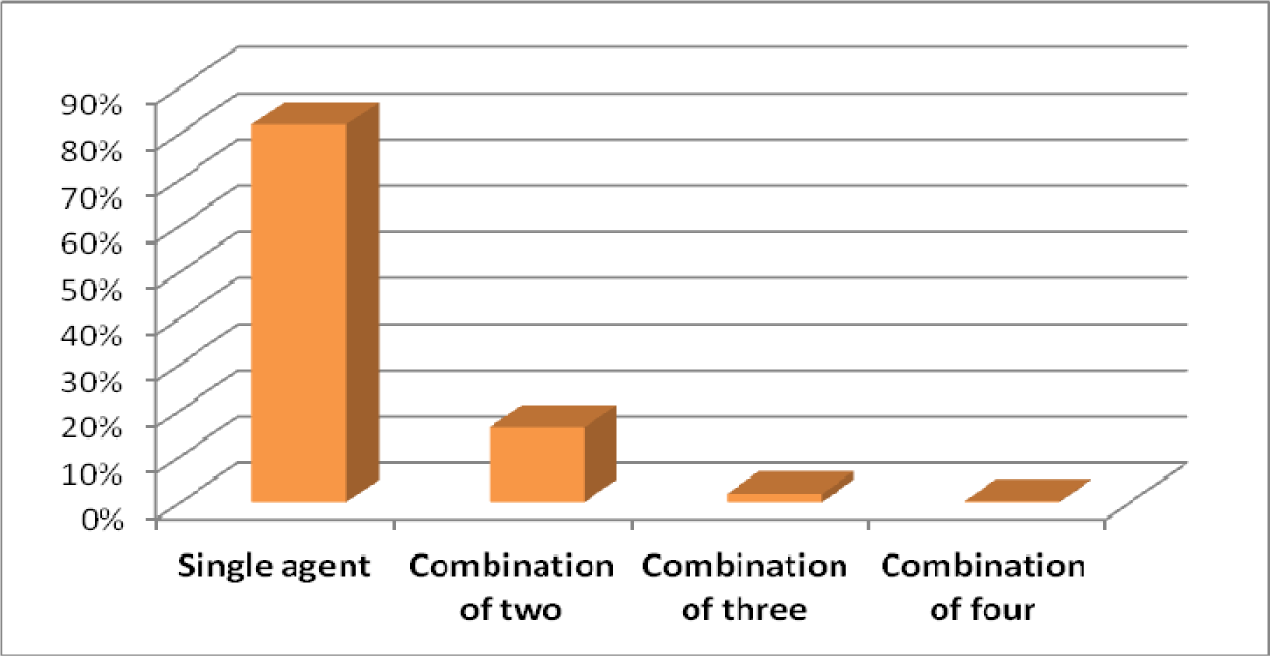
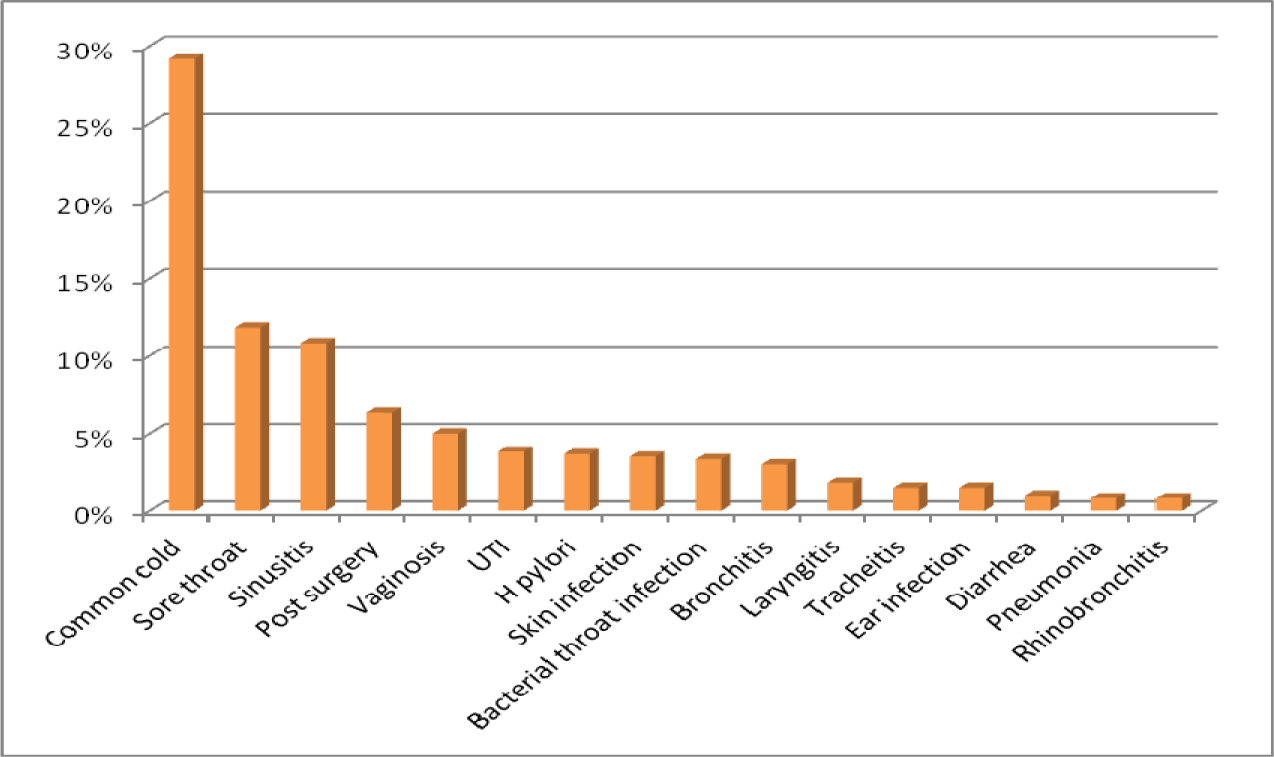
The mean age for male and female was 38.7 and 35.3 years old respectively. Patients with prescribed antibiotic for any type of infections and regardless of number of antibiotics were included in this sample size. 81.8 % of antibiotics were prescribed as a single agent, 16.2 % as combination of two, 1.8 % in combination of three and 0.2 % in combination of four (Figure 4(Fig. 4)). For the purpose of this research, six different classifications of antibiotics including Penicillins, Cephalosporins, Macrolides, Tetracyclines, Quinolones and other antimicrobial agents were considered (Table 1(Tab. 1)). The most common prescribed antibiotics were Penicillins including penicillin (18.5 %), Co-Amoxiclav (15.7 %) and Amoxicillin (4.2 %). Cephalosporins including, Cefixime (16.2 %), Cephalexin (6.7 %) as an oral formulation and Ceftriaxone as parenteral formulation (1.7 %). The Macrolides such as Azithromycin (12.8 %), Erythromycin (1.5 %) and Clarithromycin (1 %) were next in sequence. Quinolones, in specific, Ciprofloxacin usage has been increasing significantly in the recent years (Karageorgopoulos et al., 2008[11]) (Figure 5(Fig. 5)). The most common diagnosis was upper respiratory tract symptoms with common cold and sore throat with 29.2 % and 11.8 % respectively. The rest of reported diagnoses were in order; sinusitis (10.8 %), genitourinary complaints such as bacterial vaginosis and urinary tract infections (8.8 %), prevention of infection in day-case operations (included minor gastro-intestinal, cardiology, skin and urological procedures) which made 6.3 % of the total cases. Skin infections such as impetigo and cellulitis are next in the list with 3.5 % of the reported cases (Figure 6(Fig. 6)). Only based on evaluation of the directions (instructions for use), 58 % of selected antibiotics were accurate when compared against major references such as Martindale or British National Formulary. Parenteral administrations remain relatively common route of administration considering patients have been treated as an outpatient with 22 % of the reviewed prescriptions (Table 2(Tab. 2)).
Diagnoses such as common cold, sore throat and laryngitis making 42.4 % of the total diagnosis and when compared against the latest recommended therapeutic options, there is no justification for the use of antibiotics to treat the patients (Table 3(Tab. 3)). For some other diagnoses the choice of antibiotics is different to the ones recommended (inappropriate therapies). The use of Amoxicillin (4.2 %) is significantly less in comparison to co-amoxiclav (15.7 %) as many prescribers are using the combination of these two on the same prescription to get higher doses of amoxicillin and to avoid the side effects of clavulanic acid. The amoxicillin on its own is considered to have “placebo effects” by many physicians, the statement to prove that the attitude of the medical practitioners can influence the development of antibacterial resistance.
Overall, 69.8 % of prescriptions did fall short when all the factors associated with coherent prescribing of antibiotics were considered (Figure 3(Fig. 3)).
Discussion
To our knowledge this study is the only published study to tackle the prescribing habits in Iran. It highlights the extensive level of prescription with various antimicrobial agents and its association with antibacterial resistance. Antibiotic resistance is one of the global challenges that affect human health. Several factors have appeared to be the reasons for irrational prescribing. Patterns such as the use of drugs that are not linked to the diagnosis, inappropriate dose, direction and the use of antibiotics are all playing a major role in this. The lack of local and national formulary or guidelines can leave the medical professions absolutely open to the antibiotic choices and therefore this level of freedom in prescribing can add to the current problem. Furthermore, majority of antibiotics can be purchased as OTC medicines by the member of public, albeit not legally recommended (Figure 2(Fig. 2)).
Initially, the sample size of 600 items was based on the recommendation of the WHO (WHO, 2008[24]) for primary evaluation of antibiotic prescribing for population of which the demographic characteristics are as such reported by the Ministry of Health in Iran and similarly by the U.S. Census Bureau International Data Base. More than half of Iran's population is under the age of 35 years old as it was reported in 2012. In our sample size the similar pattern is observed where the male under the age of 35 is 24.2 % and the female in the same category is 30.5 % (Table 2(Tab. 2)). Overall, 65 to 70 % of antimicrobial drugs are used in outpatient settings. Taking into consideration, the key factors in the development of antimicrobial resistance such as the total amount of antimicrobial usage, class or groups of antibiotics, dosage and frequency as well as public behavior all indicates that outpatient prescribing is playing a major role in the development of antimicrobial resistance.
Most of the antibiotics were used for respiratory tract infection with 35.5 % of the total antibiotics prescribed. This is higher than UK where only 15 % are due to the similar ailments. Same as other countries we could argue that most common causes for antibiotic prescribing is due to respiratory complaints (Murphy et al., 2012[16]). However antibiotics were unjustly prescribed as suggested by the Cochrane library in 29.2 % of patients diagnosed with common cold (Table 2(Tab. 2)). Based on recommendations from National Institutes for Clinical Excellence (NICE), antibiotics should not be prescribed for all patients with upper respiratory symptoms and only should be considered in patients who are at risk of further complications such as meningitis or patients with other influential comorbidities. As it was proven in our data gathering no such important recommendations were considered when antibiotics were prescribed. Gastrointestinal antimicrobial therapy was mostly observed for eradication of Helicobacter Pylori infection for those patients who were not diagnosed in the approved manner, i.e. blind therapy and/or the choice of drug selections were inappropriate.
Researchers have previously proven in those countries where patient is paying for the consultations, medical practitioners are more sensitive to the patient's expectations and it may be more challenging for them not to prescribe the antibiotics as the patient in majority of the cases believes that he/she will need the antibiotics for the “quick recovery” (Murphy et al., 2011[17]). The antibiotic choices seem to be limited mainly to Penicillins in 40 %, Cephalosporins in 24.5 %, Macrolides in 15.3 %, Quinolones in 6.5 % and finally Tetracyclines in 2 % of the options considered (Table 2(Tab. 2)). Nevertheless, these antibiotics are listed as the main types of antibiotics which are associated with antibacterial resistance. Increased rates of Methicillin-resistant Staphylococcus aureus infections are renowned with cephalosporins and in particular with quinolones (McCusker et al., 2003[15]). Additionally, the high risk antibiotics associated with colonisation of Clostridium difficile are cephalosporins and in specific quinolones (Tacconelli et al., 2008[22]) (Figure 5(Fig. 5)).
Antibiotics are not suggested to be used for symptoms such as common cold and sore throat, however, a large number (41 %) of antibiotics were prescribed for these conditions (Figure 6(Fig. 6)). This was the major deviation from recommendations by the guidelines. In contrast, small numbers of diagnosis were recorded to be ear infections which received the antibiotics. As ear infections can be associated with more complications such as meningitis in pediatrics, majority of guidelines are more in favor of antibiotic prescribing. In other studies in excess of 89.5 % of patients with ear infections were treated with antibiotics (Murphy et al., 2011[17]) with similar trends in UK and USA (Froom et al., 2001[6]; Akkerman et al., 2005[1]) in comparison to 86 % in this study.
Routes of administrations were also investigated, with 22 % receiving parenteral therapy against 75 % who have received oral antibiotic therapy (Table 2(Tab. 2)). Bearing in mind, in outpatient setting, improper parenteral therapy with Penicillins and Ceftriaxone can have an impact on the cost of the care as well as exposing the patients to the risks associated with these routes of therapies. Ultimately, the inconvenienced caused to the patients as in many cases patients had to go back for consecutive days to complete the antibiotics prescribed. In most cases, patients do not go back to complete the course of injections and that itself is a major cause for antibiotic resistance or at least incomplete eradication of bacteria that caused the infection in the first place. Furthermore, there is no “switch therapy of antibiotics” where parenteral medications can be changed to oral, presumably, after the loading dose is given to the patients or when the patients can tolerate the oral formulations (Wiersinga et al., 2012[25]).
In comparison to the advanced countries, the uses of antibacterial guidelines are very infrequent practice as there are no local or national guidelines (Ong et al., 2008[19]). Therefore, the majority of chosen antibiotics are either random or inappropriate. This shortfall in the practice of medical professions is partly due to the education system where medical graduates are not familiar with the guidelines and or wrong prescribing habits due to insufficient prescribing trainings in undergraduate's syllabus.
The risk of reduction or cessation of antibiotic effectiveness is influenced by this world wide problem (Vazquez-Lago et al., 2011[23]; Ashiru-Oredope et al., 2012[3]; Neu, 1992[18]; Hogerzeil, 1995[10]). According to the WHO report, among Asian countries, Indonesia, Nepal and Bengladesh have the most patients receiving antibiotic, respectively. Regardless, Iran is one of the countries with high antibiotic consumption, but in global statistics there isn't any report of antibiotic consumption in Iran. Therefore, we were prompted to carry out an investigational research on antibiotic prescribing pattern in Iran. This research is a pilot study and our research team is expanding the current work on comprehensive level in Iran. Results of this study publicized the wide range of issues associated with the current use of antibiotics in the largest teaching hospitals, training the biggest number of medical graduates and due to its broad extent of distribution cover the largest number of population in the capital city Tehran.
The antibiotic prescribing was unjustified in 42.7 % of the cases. The prescribing habit, correct diagnosis and the use of antibiotics need instant consideration. Additionally, it is tremendously important to increase the public knowledge concerning appropriate use of antibiotics and consequently reduce the pressure on the prescribers.
These data can provide useful information for assessing public-health policy in Iran or indeed any other country with the same pattern of antibiotic prescribing in order to reduce the antibiotic use and the level of resistance. The author believes similar studies ought to take place in other countries with the same challenges in order to deal with this global and growing problem. On the other hand, such challenges cannot be fully addressed without the support of the WHO and sharing the experiences with the developed countries.
Acknowledgements
We would like to thank all medical, clinical and nursing teams who have supported this research throughout.
Declaration
Dr Mehdi Rajabi is a visiting lecturer, a member of General Pharmaceutical Council of Great Britain and also a freelance clinical pharmacist in various teaching hospitals in UK.
Conflict of interest
None.
References
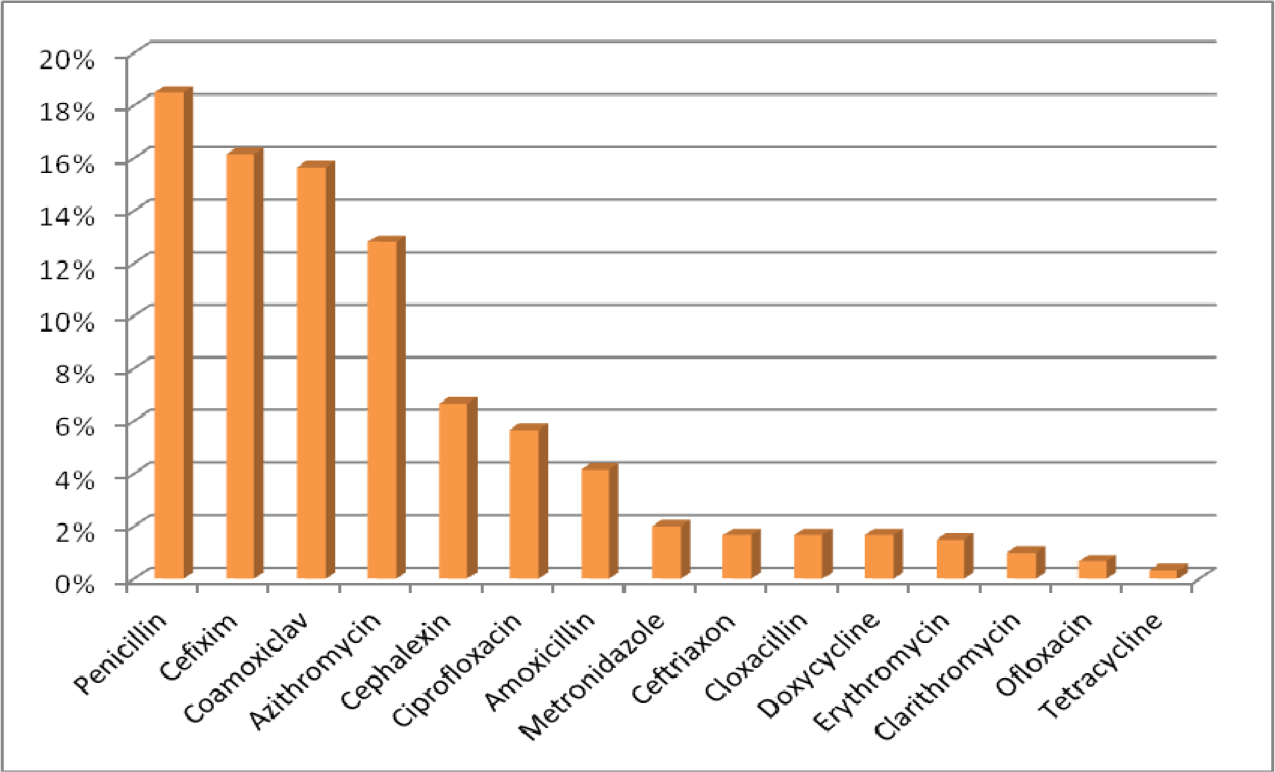
Figure 5: Detailed fraction of the most common antibacterial agents prescribed in outpatient centers
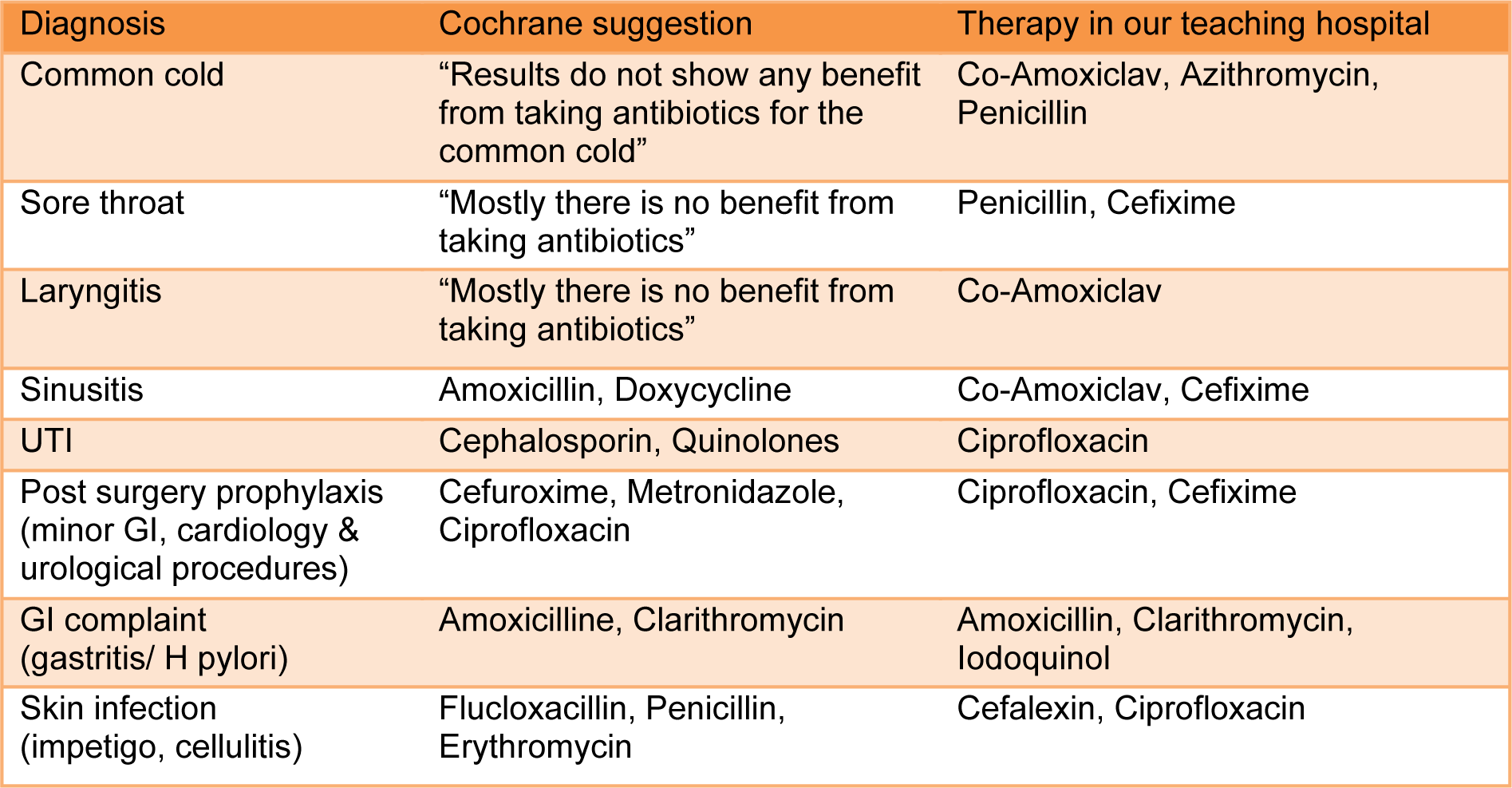
Table 3: Comparison between antibacterial therapies in teaching hospitals against Cochrane suggestion
[*] Corresponding Author:
Dr. Mehdi Rajabi, Department of Clinical Pharmacy, Islamic Azad University Pharmaceutical Sciences Branch, Tehran, Iran, eMail: Mehdirj@aol.co.uk