Research article
Heme oxygenase effect on mesenchymal stem cells action on experimental Alzheimer's disease
MT Abdel Aziza1, HM Atta2, H Samer3, HH Ahmed1, LA Rashed1, D Sabry1, ER Abdel Raouf4, Marwa Abdul latif Alkaffas1[*]
1Department of Medical Biochemistry, Unit of Biochemistry and Molecular Biology, Faculty of Medicine, Cairo University, Cairo, Egypt2Clinical Biochemistry Department, King Abdulaziz University, Jeddah, Saudi Arabia
3Department of Neurology, Faculty of Medicine, Cairo University, Cairo, Egypt
4Department of Pediatrics, National Research Center
EXCLI J 2013;12:Doc778
Abstract
The objective is to evaluate the effect of heme oxygenase-1 (HO-1) enzyme inducer and inhibitor on Mesenchymal Stem Cells (MSCs) in Alzheimer disease. 70 female albino rats were divided equally into 7 groups as follows: group 1: healthy control; group 2: Aluminium chloride induced Alzheimer disease; group 3: induced Alzheimer rats that received intravenous injection of MSCs; group 4: induced Alzheimer rats that received MSCs and HO inducer cobalt protoporphyrin; group 5: induced Alzheimer rats that received MSCs and HO inhibitor zinc protoporphyrin; group 6: induced Alzheimer rats that received HO inducer; group7: induced Alzheimer rats that received HO inhibitor. Brain tissue was collected for HO-1, seladin-1 gene expression by real time polymerase chain reaction, heme oxygenase activity, cholesterol estimation and histopathological examination. MSCs decreased the plaque lesions, heme oxygenase induction with stem cells also decreased plaque lesions however there was hemorrhage in the brain. Both heme oxygenase inducer alone or with stem cells increased seladin-1 expression and decreased cholesterol level. MSCs alone or with HO-1 induction exert a therapeutic effect against the brain lesion in Alzheimer's disease possibly through decreasing the brain cholesterol level and increasing seladin-1 gene expression.
Keywords: Alzheimer's disease, stem cell, heme oxygenase
Introduction
Alzheimer's disease is a progressive neurodegenerative disease characterized by abnormal clumps (amyloid plaques) and tangled bundles of fibers (neurofibrillary tangles) composed of misplaced proteins in the brain. It is manifested by a progressive decline in cognitive abilities such as memory, comprehension, language expression, learning capacity, calculation, abstraction, judgment, spatial orientation and the recognition of familiar people or place (Berchtold and Cotman, 1998[5]). Neurologically, there are 3 main identifying features of Alzheimer's disease: beta-amyloid plaques which form outside and around neurons, neurofibrillary tangles which form inside dead neurons and overall dramatic shrinkage of neural tissues. The “plaques” and “tangles” in particular have come to be regarded as the hallmarks of Alzheimer's disease (Brookmeyer et al., 1998[7]). Age is considered to be the greatest “risk factor” for Alzheimer's, as the number of people diagnosed with the disease doubles for every 5 years beyond the age 65. On the other hand, approximately 5 % of men and 6 % of women have developed Alzheimer's by the age of 65 (Bodles et al., 2001[6]; Quinlan et al., 2001[28]).
The precise causes of Alzheimer's disease are believed to involve a complex interaction of genetic and environmental factors, as no one particular etiology has been identified that would explain all cases. Since there is no recognized cure for Alzheimer's disease, conventional medical treatment has typically consisted primarily of drug therapy which is prescribed with the hope of managing symptoms, although such medication does not alter the progression of the disease itself. Medications fall into a class of drugs known as cholinesterase inhibitors (Bodles et al., 2001[6]; Quinlan et al., 2001[28]).
As a chronic, progressive, neurodegenerative disorder, Alzheimer's disease is a prime candidate for adult stem cell treatment. Indeed, research has shown a variety of promising approaches to the treatment of this disease with adult stem cells. Alzheimer's disease is characterized by the destruction of neuronal circuitry which is the result of a buildup of beta-amyloid plaques in combination with the tangles that form the tau protein. An effective treatment for Alzheimer's disease, therefore, would be one which would prevent and reverse the formation of such plaques and tangles, while also regenerating lost neuronal connections. Thus far, the only therapy that offers the possibility of accomplishing these objectives is stem cell therapy (Lie et al., 2004[22]; Ke et al., 2006[19]; Danilov et al., 2006[11]).
Mesenchymal stem cells (MSCs) are pluripotent cells isolated from the bone marrow and other various organs. They are able to proliferate and self-renew, as well as to give rise to progeny of at least the osteogenic and adipogenic lineages (Weissman, 2000[39]). Indeed, research has shown a variety of promising approaches to the treatment of this disease with adult stem cells (Chen et al., 2000[9]).
The heme oxygenase (HO) enzyme catalyses the degradation of heme. It was reported that the activity of HO is reduced in familial or early onset Alzheimer disease. The products of the reaction include biliverdin and carbon monoxide; they are all involved in various ways in the pathway of oxidative stress. Biliverdin is a potent antioxidant agent. Under normal condition biliverdin is metabolized by biliverdin reductase to bilirubin that also decreases oxidative impairment of the cells. CO is a gaseous material that activates guanyl cyclase increasing cyclic guanosine monophosphate (cGMP) which acts as neurotransmitter and vasodilator (Takahashi et al., 2000[31]). HO-1 plays a vital role in many aspects, such as suppression of oxidative stress, inflammation, cell proliferation and regulation of cytokine expression in various pathological conditions (Eugenio Barone et al., 2011[3]).
This work aims to study the effect of heme oxygenase enzyme inducer and inhibitor on stem cells in neurodegenerative lesions of Alzheimer disease.
Materials and Methods
Preparation of the animal model
Experimental animals
The study was carried on 70 female white albino rats weighing 150 to 200 g. Rats were bred and maintained in an air-conditioned animal house with specific pathogen-free conditions, and were subjected to a 12:12-h daylight/darkness and allowed unlimited access to chow and water. All the ethical protocols for animal treatment were followed and supervised by the animal facilities, Faculty of Medicine, Cairo University. All animal experiments received approval from the Institutional Animal Ethics Committee. They were divided into 7 groups as follow:
- Group 1: 10 rats as a healthy control group
- Group 2: 10 rats as Aluminium chloride induced Alzheimer disease (17 mg/kg b. wt daily for 75 days) (Krasovskiĭ et al., 1979[20])
- Group 3: 10 induced Alzheimer rats that received MSCs only, which were processed and cultured for 14 days, in a single dose of 106 cells per rat, given by intravenous infusion in the rat tail vein .The rats were kept for one month.
- Group 4: 10 induced Alzheimer rats that received MSCs and HO inducer, cobalt protoporphyrin in a single dose of (0.5 mg/100 gm) subcutaneously. The rats were kept for one month.
- Group 5: 10 induced Alzheimer rats that received MSCs and HO inhibitor, zinc protoporphyrin in a single dose of (10 mmol/kg) subcutaneously. The rats were kept for one month.
- Group 6: 10 induced Alzheimer rats that received HO inducer.
- Group 7: 10 induced Alzheimer rats that received HO inhibitor.
After 75 days, 5 animals were scarified and pathological examination was done to ensure Alzheimer disease induction.
Brain tissues were dissected and examined for:
- Quantitative analysis of heme oxygenase-1 gene and seladin-1 gene expression by real time PCR, heme oxygenase activity and cholesterol estimation.
- Histopathological examination of brain tissue by hematoxylin and eosin.
- Detection of the MSCs homing in brain tissue after its labeling with PKH26 dye by fluorescence microscopy to detect its red fluorescence.
Preparation of BM-derived mesenchymal stem cells from rats
Bone marrow was harvested by flushing the tibiae and femurs of 6-week-old male white albino rats with Dulbecco's modified Eagle's medium (DMEM, GIBCO/BRL) supplemented with 10 % fetal bovine serum (GIBCO/BRL). Nucleated cells were isolated with a density gradient [Ficoll/Paque (Pharmacia)] and resuspended in complete culture medium supplemented with 1 % penicillin-streptomycin (GIBCO/BRL). Cells were incubated at 37 °C in 5 % humidified CO2 for 12-14 days as primary culture or upon formation of large colonies. When large colonies developed (80-90 % confluence), cultures were washed twice with phosphate buffer saline (PBS) and the cells were trypsinized with 0.25 % trypsin in 1 mM EDTA (GIBCO/BRL) for 5 min at 37 °C. After centrifugation, cells were resuspended in serum-supplemented medium and incubated in 50 cm2 culture flask (Falcon). The resulting cultures were referred to as first-passage cultures (Abdel Aziz et al., 2007[1]). MSCs in culture were characterized by their adhesiveness and fusiform shape. Also CD29 gene expression was detected by RT-PCR as a marker of MSCs (Abdel Aziz et al., 2007[1]).
Labeling of MSCs with PKH26
MSCs were harvested during the 4th passage and were labeled with PKH26, which is a red fluorochrome. The fluorochrome has excitation (551 nm) and emission (567 nm) characteristics compatible with rhodamine or phycoerythrin detection systems. The linkers are physiologically stable and show little to no toxic side-effects on cell systems. Labeled cells retain both biological and proliferating activity, and are ideal for in vitro cell labeling, in vitro proliferation studies and long, in vivo cell tracking. In the current work, MSCs were labeled with PKH26 from Sigma Company (St. Louis, MO/USA). Cells were centrifuged and washed twice in serum free medium. Cells were pelleted and suspended in dye solution. Cells were injected intravenously into rat tail vein. After one month, liver tissue was examined with a fluorescence microscope to detect and trace the cells.
Real-time quantitative analysis for heme oxygenase-1 and seladin-1 gene expression
Total RNA was extracted from brain tissue homogenate using RNeasy purification reagent (Qiagen, Valencia, CA). cDNA was generated from 5 μg of total RNA extracted with 1 μl (20 pmol) antisense primer and 0.8 μl superscript AMV reverse transcriptase for 60 min at 37 °C.
The relative abundance of mRNA species was assessed on an ABI prism 7500 sequence detector system (Applied Biosystems, Foster City, CA). PCR primers were designed with Gene Runner Software (Hasting Software, Inc., Hasting, NY) from RNA sequences from GenBank (Table 1(Tab. 1)). All primer sets had a calculated annealing temperature of 60°. Quantitative RT-PCR was performed in duplicate in a 25-μl reaction volume consisting of 2X SYBR Green PCR Master Mix (Applied Biosystems), 900 nM of each primer and 2-3 μl of cDNA. Amplification conditions were 2 min at 50°, 10 min at 95° and 40 cycles of denaturation for 15 s and annealing/extension at 60° for 10 min. Data from real-time assays were calculated using the v1·7 Sequence Detection Software from PE Biosystems (Foster City, CA). Relative expression of VEGF, TNF alpha and IL10-mRNA was calculated using the comparative Ct method as previously described. All values were normalized to the β-actin gene and reported as fold change over background levels detected in Alzheimer Disease.
Biochemical analysis
Brain tissue was homogenized and centrifugated at 3000 °C for 10 minutes. The supernatant was collected and kept frozen at -80 °C till analysis of cholesterol by using a commercially available kit.
Analysis of brain histopathology
The obtained tissue sections were collected on glass slides, deparaffinized, stained by hematoxylin and eosin. Examination was performed by light microscopy (Bancroft et al., 1996)[2].
HO activity assay
Brain tissue was homogenized with 2.5 volume Tris-HCl buffer (10 mmol/L, pH 7.6) containing 250 mmol/L sucrose and 0.4 mmol/L phenylmethylsulfonyl fluoride. The homogenate was centrifuged at 800 g for 10 minutes and then at 13500 g for 20 minutes to produce the mitochondrial pellet. The supernatant was withdrawn. The protein content was determined by the method of Lowry et al. (1951[23]). The activity of HO in the supernatant was determined as follows; the supernatants were incubated at 37 °C for 1 hour with heme (50 mmol/ L), rat liver cytosol (5 mg/mL), MgCl2 (2 mmol/L), glucose-6-phosphate dehydrogenase (1 unit), glucose-6-phosphate (2 mmol/L), and NADPH (0.8 mmol/L) in 0.5 mL of 0.1 mol/L phosphate buffer saline (pH 7.4). The reaction was stopped by putting the tubes on crushed ice, then the bilirubin generated was extracted by chloroform and its amount was determined with a scanning spectrophotometer and was defined as the difference between the absorbance at 463 and 520 nm, while using a standard bilirubin curve.
Statistical analysis
Data were expressed as mean ± SD. Significant differences were determined by using ANOVA and post-hoc tests (LSD) for multiple comparisons using SPSS 9.0 computer Software. Results were considered significant at p<0.05.
Results
MSCs culture, identification & homing
Isolated and cultured undifferentiated MSCs reached 70-80 % confluence in 14 days. In vitro osteogenic and chondrogenic differentiation of MSCs were confirmed by morphological changes and special stains (Figure 1A, B(Fig. 1) and Figure 2A, B(Fig. 2) respectively). In addition MSCs were identified by surface marker CD29 (+) by PCR (Figure 3(Fig. 3)). MSCs labeled with PKH26 fluorescent dye were detected in the brain tissues confirming that these cells homed into the brain tissues (Figure 4(Fig. 4)).
MSCs and/or HO improve the neurodegenerative lesions in the brain
The results of the present study show a significant decrease in the cholesterol level and HO activity in AD/MSC, AD/MSCs/ HO inducer groups compared to the AD group (P<0.05) (Table 2(Tab. 2)).
Gene expression of heme oxygenase-1, seladin-1 genes
Concerning gene expression, there was a significant increase in the heme oxygenase-1expression and decrease in the seladin-1 gene expression in the AD group compared to the control group (P<0.05). Following MSC injection, the HO-1 expression was insignificantly increased (P=1.000), while seladin-1 expression increased significantly (P<0.05) compared to the AD group. Following MSC injection with HO inducer, the HO-1 expression increased significantly (P<0.05) compared to the AD group and AD with MSCs, the Seladin-1 expression increased significantly (P<0.05) compared to the AD group but insignificantly compared to AD with MSCs group (P=1.000). Following MSC injection with HO inhibitor, H0-1 expression decreased significantly (P<0.05) compared to the AD group, AD with MSCs and AD with MSCs with HO inducer group while the seladin-1 expression increased significantly (P<0.05) compared to the AD group, AD with MSCs group and AD with MSCs with HO inducer group. Following HO inducer, the HO-1 expression increased significantly (P<0.05) compared to all other groups while the seladin-1 expression decreased significantly (P<0.05) compared to the AD with MSC, AD with MSCs with HO inducer group, AD with MSCs with HO inhibitor group but was insignificantly decreased compared to the AD group (p=0.923). Following HO inhibitor, the HO-1 expression decreased significantly (P<0.05) compared to all other groups while the seladin-1 expression increased significantly (P<0.05) compared to the AD with MSCs, AD with MSCs with HO inducer and the AD with MSC with HO inhibitor group, but was insignificantly decreased compared to AD group (p=0.949) and the AD with HO inducer group (P=0.872 (Figure 5A, B(Fig. 5))).
Histopathological examination of brain tissues in different groups
Histopathological examination of the brain tissue of the AD group showed multiple acellular plaques in the mid brain, associated with oedema, hypoplasia, and congested blood capillary in the hippocampus (Figure 6B(Fig. 6)). Following MSCs injection there was congestion in the blood vessels and focal gliosis in the cerebral cortex (Figure 6C(Fig. 6)).With MSCs & HO inducer there was congestion in the meninges, associated with focal hemorrhage and oedema with gliosis in the hippocampus (Figure 6D(Fig. 6)). With MSCs & HO inhibitor there was diffuse gliosis in the cerebral cortex, associated with focal hemorrhage in the brain stem (Figure 6E(Fig. 6)). After injection of the inducer alone there was neuronal degeneration in the brain stem associated with focal gliosis in the cerebrum and congestion with hemorrhage in the hippocampus (Figure 6F(Fig. 6)). After injection of the inhibitor alone focal gliosis with plaque formation were observed in the cerebrum, associated with atrophy and gliosis in the hippocampus (Figure 6G(Fig. 6)).
Discussion
Bone marrow-derived stem cells contribute to cell turnover and repair in various tissue types, including the brain (Cornacchia et al. 2001[10]; Poulsom et al., 2001[26]). MSCs are commonly defined as bone marrow-derived fibroblast-like cells, which despite the lack of specific surface markers can be selected by their adherence characteristics in vitro and which have the ability to differentiate along the three principal mesenchymal lineages: osteoblastic, adipocytic and chondrocytic (Pittenger et al., 1999[25]; Imai and Ito, 2002[15]). Studies demonstrated that MSCs are non-immunogenic and display immunosuppressive properties, with the ability to inhibit maturation of dendritic cells and to suppress the fraction of memory T cells, B cells and Natural Killer (NK) cells. They also possess transdifferentiation and antiapoptotic ability. These properties of MSCs render these cells especially attractive for therapeutic application in several inflammatory and neurodegenerative disease, as well as in regenerative medicine (Togel et al., 2007[34]). In the present study, bone marrow derived mesenchymal stem cells were isolated from male rats, grown and characterized by their adhesiveness and fusiform shape and by detection of CD 29, one of surface marker of rat mesenchymal stem cells. These MSCs were used to detect their possible anti-inflammatory and transdifferentiation role in amelioration of brain lesions (plaques) in the experimental model of Alzheimer's disease. We also tested whether MSCs alone or MSCs with heme oxygenase inducer could improve brain lesions (plaques) in experimental model of Alzheimer's disease.
It was hypothesized that BM-MSCs transplantation may have beneficial effects in AD patients through playing a specific and a general role in Alzheimer's disease. The specific role is through microglial activation. Microglia cells are resident in the Central Nervous System (CNS) that regulate innate immunity and participate in adaptive immune response in CNS tissues. Microglia has a neuroprotective function by secreting neurotrophic agents (Nguyen et al., 2002[24]) and eliminating Aβ via phagocytosis. It was suggested that transplantation of BM-MSCs into the brain promotes the clearance of Amyloid Beta (AB) deposits and/or blocks their formation, and that microglia/macrophage recruitment which was found to be enhanced by grafted Bone Marrow-MSCs (BM-MSCs) might be involved in this process. Experimental AD model was induced by injecting amyloid-β (AB) into the dentate gyrus (DG) of hippocampus of mice. Intracerebral transplantation of BM-MSCs into the brain of an induced AD model reduced their AB levels when compared to control animal (Trzaska et al., 2008[35]). Also through its transdifferentiation ability; several studies have demonstrated that MSCs can generate cells of neuroectodermal origin (Trzaska et al., 2008[35]). This generation of ectodermal neurons is termed transdifferentiation, or plasticity. Although the plasticity of MSCs is still controversial, many more studies are confirming that they can transdifferentiate or 'jump germ layers' (Zipori, 2004[42]). Under various culture conditions and stimulation with inductive factors, MSCs exhibit vast plasticity and are capable of generating several cell types outside of their mesodermal origin. Transplantation of MSCs into the subventricular zone (SVZ) of mice housed in an enriched environment stimulates cell proliferation and neuronal differentiation. Additional studies showed that MSCs promote endogenous neurogenesis in the hippocampus ((Tfilin et al., 2009[33]; Kan et al., 2011[18]). Finally through secreting neurotrophic factors of nutritional value as brain derived neurotrophic factor (BDNF) and glial derived neurotrophic factor (GDNF) either by itself or through the microglia cells that support the regenerative microenvironment (Caplan, 2007[8]). The general role may be through its anti-apoptotic role or its immunomodulatory role via suppression of T cells, inhibition of B-cell proliferation and suppress NK cells activation (Gerdoni et al., 2007[13]).
In this study, the histopathological examination of brain tissue showed no plaques but the cerebral cortex showed congestion in the blood vessels and focal gliosis. These findings agree with Lee et al. (2009[21]), who stated that BM-MSCs can promote the reduction of Aβ through the microglia activation in induced AD brain suggesting a potential therapeutic agent against AD. These findings also agree with Lee et al. (2009[21]), who stated that transplanted BM-MSCs caused reduction in Aβ in induced AD mice.
Another possible mechanism may be through decreasing cholesterol level and increasing seladin-1 expression. Seladin-1 (SELective AD INdicator-1) which is a novel neuroprotective gene, found to be down regulated in brain lesions in AD. This result was in accordance with Benvenuti et al. (2006[4]), who found that the hippocampus and the subventricular zone, which are affected in AD, are the unique regions containing stem cells with neurogenic potential in the adult brain. It might be hypothesized that this multipotent cell compartment is the predominant source of seladin in normal brain. After isolation and characterization of mesenchymal stem cells (MSCs) as a model of cells with the ability to differentiate into neurons. MSCs were then differentiated toward a neuronal phenotype (MSC-n). These cells were thoroughly characterized and proved to be neurons, as assessed by molecular and electrophysiological evaluation. Seladin-1 expression was determined and found to be significantly reduced in MSC-n compared to undifferentiated cells. These changes in seladin-1 gene expression could be attributed for the antiapoptotic function exerted by conferring resistance against oxidative stress (Di Stasi et al., 2005[12]).
Heme oxygenase has a cytoprotective, anti-apoptotic and anti-inflammatory action. Its role in Alzheimer's disease may be through facilitating phagocytosis, Exogenous HO-1 protein administration was shown to induce the production of cytokines, Tissue necrotic Factor (TNFα) and Interleukin (IL-6), and facilitate the phagocytosis and clearance of amyloid-β peptides in rat and mouse microglia mediated by Toll like Receptor (TLR4) activation (Kakimura et al., 2002[17]). These results suggest that HO-1 may have a role to play in neuroprotection in the brain. Another role may be via secreting brain derived neurotrophic factor and glial derived neurotrophic factor expression. Hung et al. (2010[14]) found that HO-1 induction by adenovirus containing human HO-1 gene (Ad-HO-1) in substantia nigra of rat increases BDNF and GDNF expression. On the other hand, glial HO-1 hyperactivity may contribute to cellular oxidative stress, pathological iron deposition, and the bioenergetic failure characteristics of degenerating and inflamed neural tissues and thus may constitute a rational target for therapeutic intervention in these conditions (Song et al., 2006[30]). Also it may be through stimulating Tau degradation. The ubiquitin-proteosome system (UPS) mediates turnover of normal, mis-folded and chemically modified (e.g. oxidized) intracellular proteins. Tau protein is degraded by the UPS in vitro and in vivo. Proteosomal activity is reduced in AD brain and b-amyloid peptide inhibits the UPS in cultured cells (Shastry, 2003[29]). Expression of HO-1 by transient transfection triggers proteosomal tau degradation in human neuroblastoma cells, an effect that can be blocked with SnMP or the proteosomal inhibitor, lactacystin. Up-regulation of HO-1 in AD brain (Premkumar et al., 1995[27]) may therefore serve to stimulate a failing UPS in an attempt to limit the accumulation of toxic tau aggregates. Interestingly, HO-1 may keep tau levels in check by more than one mechanism as Takeda et al. (2000[32]) reported transcriptional suppression of tau in neuroblastoma cells transfected with human HO-1 cDNA. The general role of HO may be through its cytoprotective, anti-apoptotic and anti-inflammatory action via decrease in caspase activity and cytochrome c release. Also it is reported that HO-1 overexpression reduces heme induced intercellular adhesion molecule-1 (ICAM-1) expression, whereas inhibition of HO activity increases heme-induced ICAM-1 expression and leukocyte influx (Wagener et al., 2001[38]).
The role of heme oxygenase with stem cells had been reported on cardiac performance. Induction of human HO-1 expression (human heme oxygenase-1, hHO-1, was transfected into cultured MSCs using an adenoviral vector) in ischemic myocardium increasesd MSCs survival and aided MSCs immunomodulatory function via reduction of TNF-α, IL-1-beta and IL-6-mRNA expression, increased IL-10-mRNA expression and significant decrease in proapoptotic Bcl-2-associated X protein (Bax) expression (Zeng et al., 2008[41]).
In the present study, heme oxygenase induction with stem cells in histopathological examination of brain tissue revealed no plaques but hemorrhage and congestion were noticed in the meninges, associated with focal hemorrhage in the brain stem, and edema with gliosis in the hippocampus. The possible mechanism may be through decreasing brain cholesterol level. Xiong et al. (2008[40]) concluded that cholesterol homeostasis and transport was impaired in AD, leading to increased retention in AD brains, due to altered levels or activities of nuclear receptors. Vaya and Schipper (2007[36]) also concluded that in advanced AD, a massive increase in the free cholesterol pool (derived from widespread neuronal degeneration) saturates sterol efflux mechanisms resulting in increased brain cholesterol levels which, in turn, exacerbate amyloid deposition and neurodegeneration in AD. Infante et al. (2010[16]) concluded through studying the polymorphism in the promoter region of liver X receptor beta (LXR-B), that heme oxygenase-1 (HO-1) stimulates oxidation of glial cholesterol to oxysterols, and increased oxysterol concentrations may protect neural tissues by activation of liver X receptor-β (LXR-β), which induces transcription of genes associated with reduction of cellular cholesterol concentrations and decrease of Aβ formation. In cultured rat astrocytes, HO-1 augments intraglial oxidative stress and promotes the conversion (oxidation) of cholesterol to oxysterols (Vaya et al., 2007[37]).
In conclusion, these data revealed that infusion of HO inducer either alone or associated with BM-derived MSCs exert a therapeutic effect on the brain lesion in Alzheimer's disease possibly through decreasing the brain cholesterol level and increasing seladin-1 gene expression.
Acknowledgement
This work was performed in the Unit of Medical Biochemistry and Molecular Biology, Biochemistry Department, Faculty of Medicine, Cairo University.
References
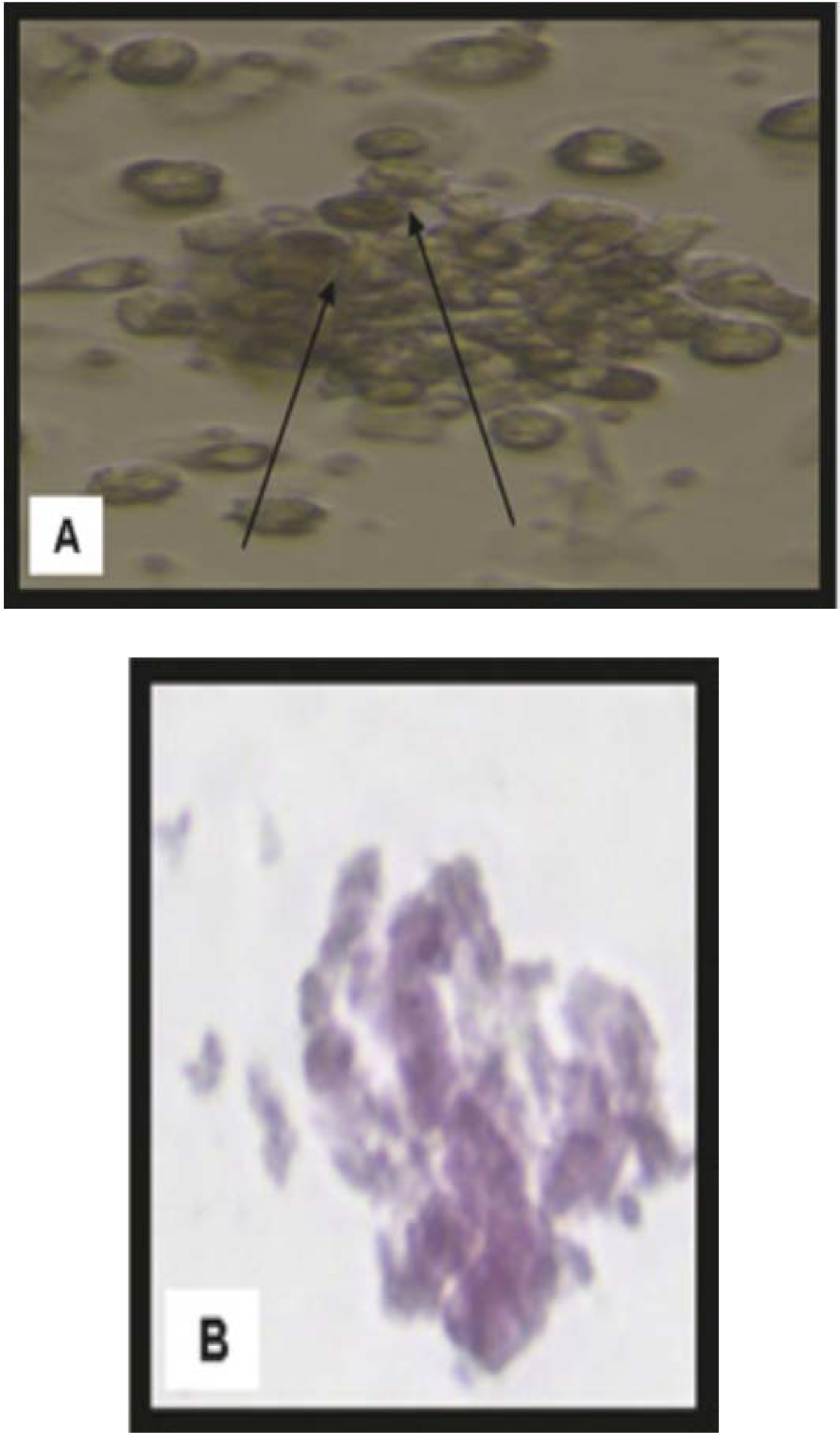
Figure 1: Morphological and histological staining of BM-MSCs differentiated into osteoblasts
(A) (×20) Arrows for differentiated MSCs osteoblasts after addition of growth factors
(B) (×200) MSCs differentiated into osteoblasts stained with Alizarin red stain
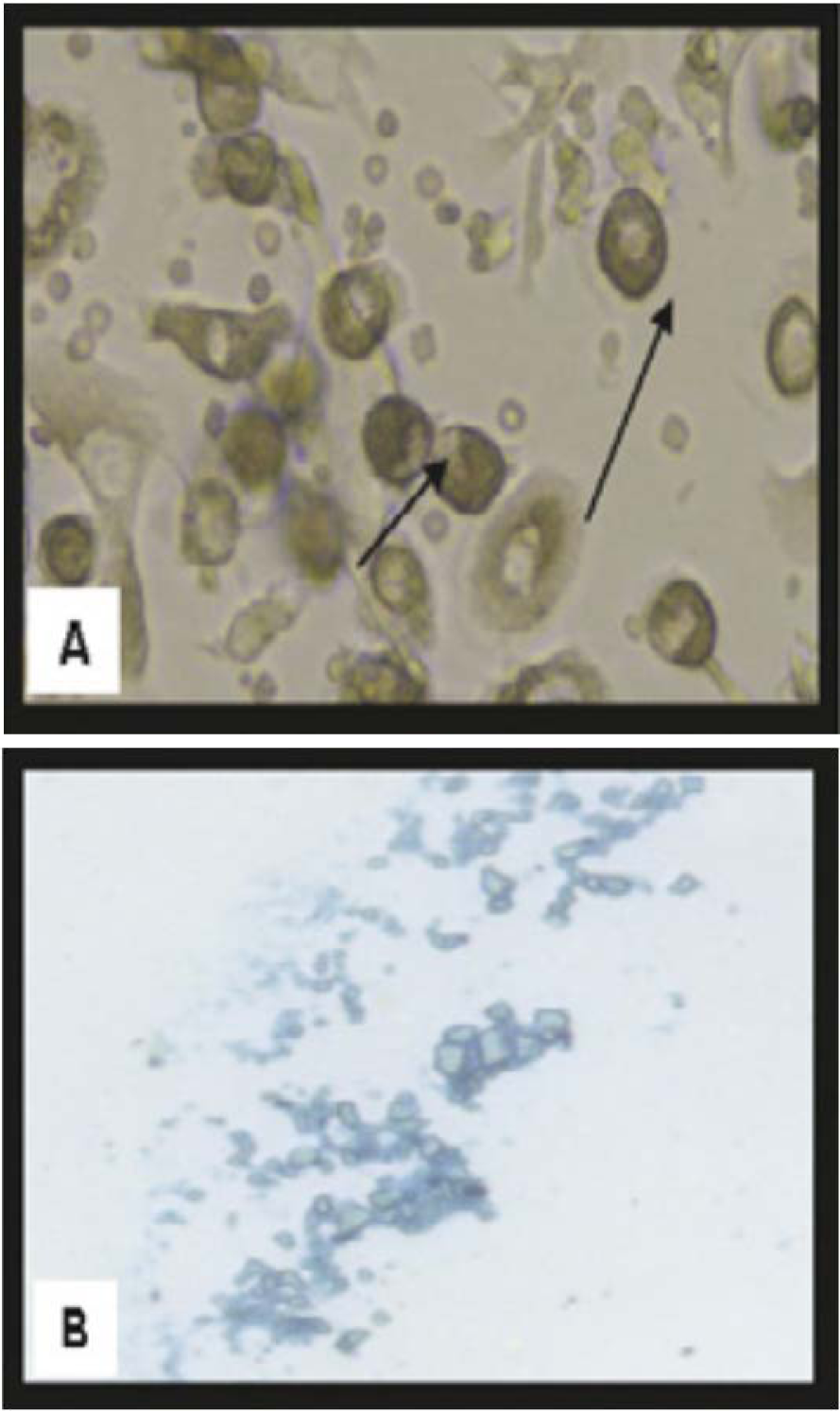
Figure 2: Morphological and histological staining of BM-MSCs differentiated into chondrocytes
(A) (×20) Arrows for differentiated MSCs chondrocytes after addition of growth factors
(B) (×200) MSCs differentiated into chondrocytes stained with Alcian blue stain
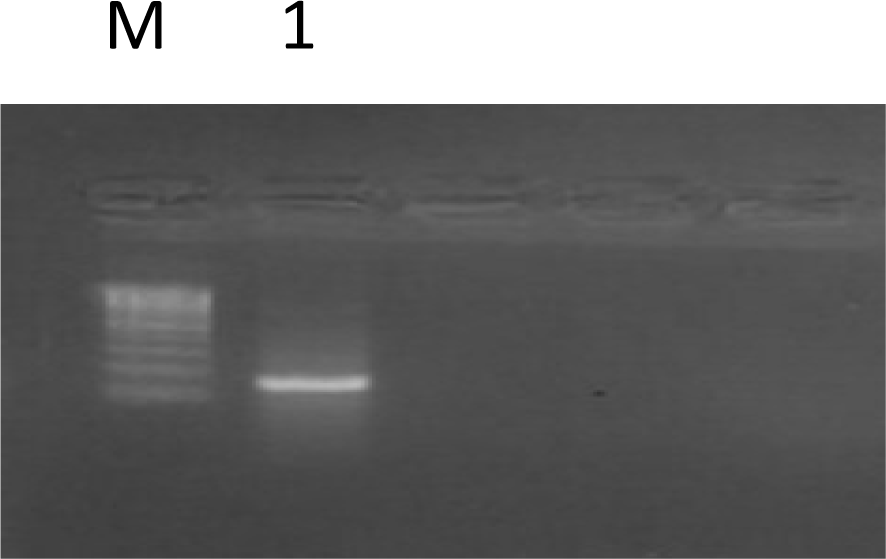
Figure 3: An agarose gel electrophoresis shows PCR of CD29 gene expression in MSC culture (261 bp) (as a molecular marker for rat MSCs)
Lane M: DNA marker with 100 bp;
Lane 1: MSCs culture
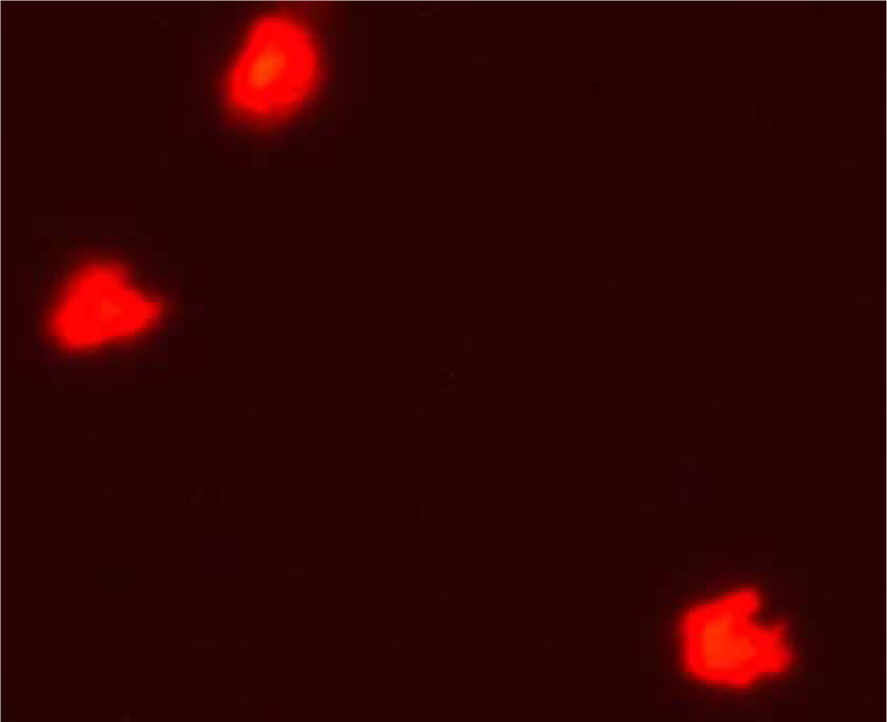
Figure 4: Labeling of MSC with PKH26 dye
Cells labeled with the PKH26 showed strong red autofluorescence after transplantation in rats, confirming that these cells were actually seeded into the brain tissue.
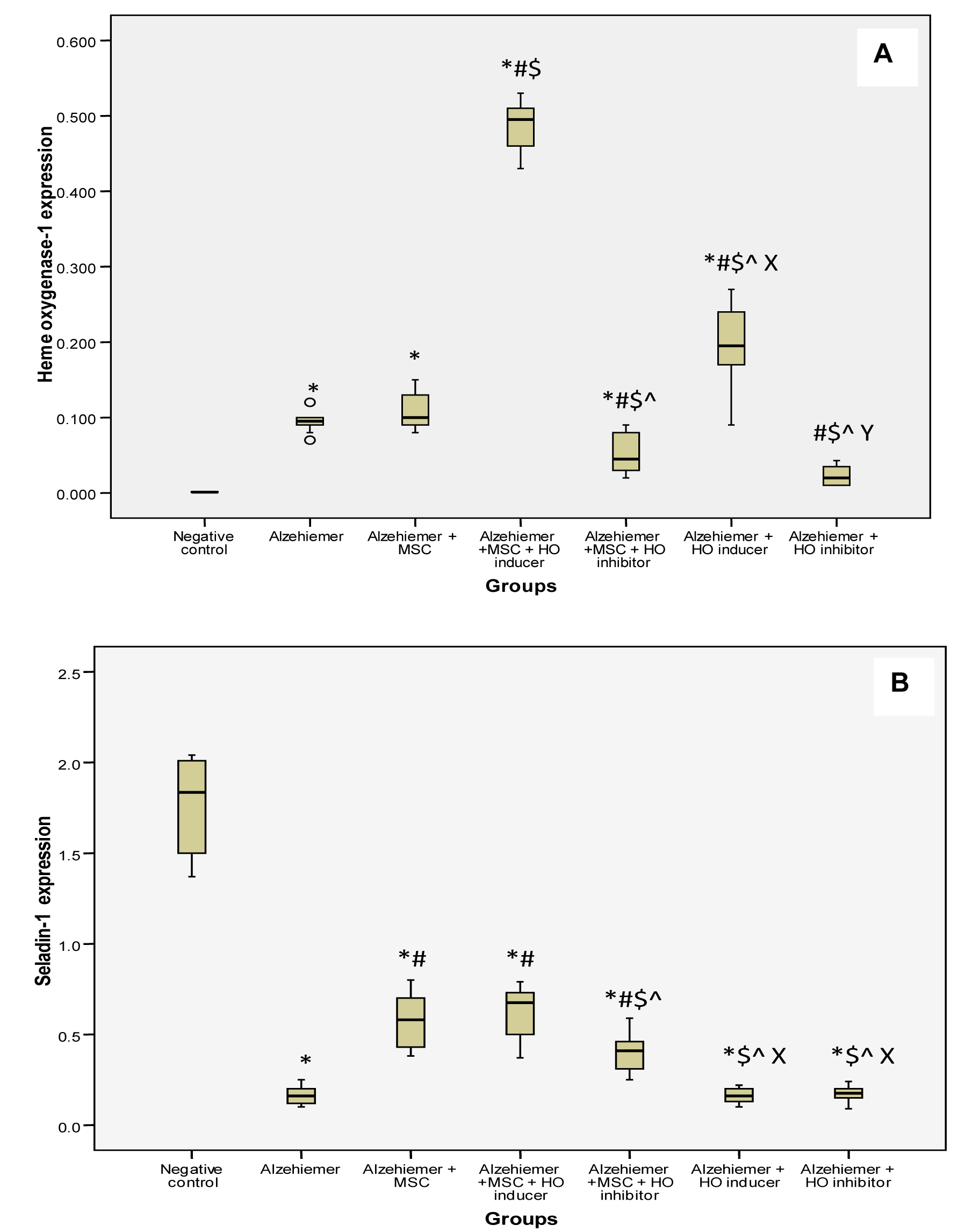
Figure 5: A) Box plots analysis of heme oxygenase-1, B) Box plots analysis of seladin-1 gene expression by real time PCR in different groups
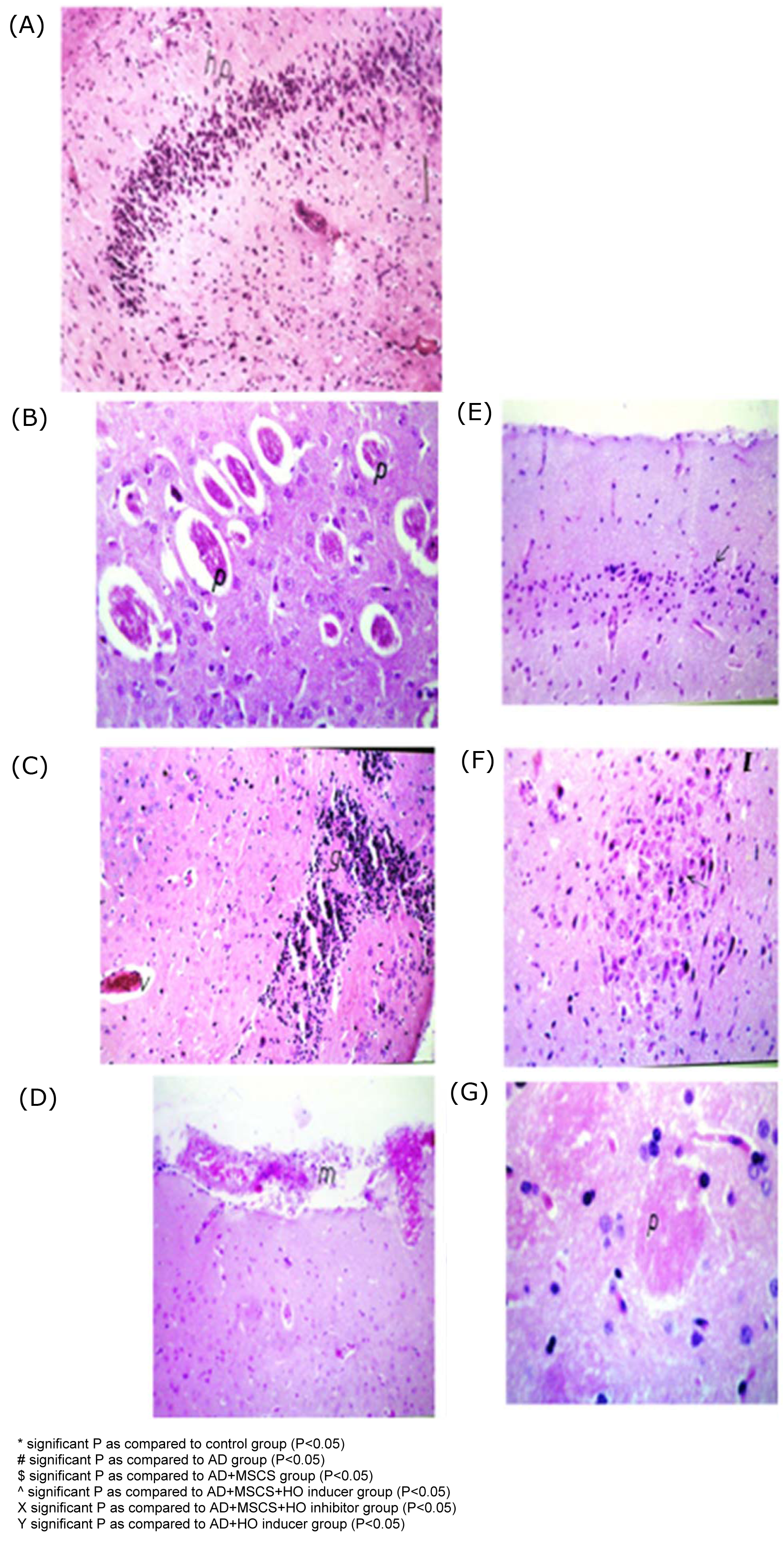
Figure 6: Histopathological examination of brain tissues in different groups
(A) Brain of control rat showing intact histological structure of the hippocampus
(B) AD (H&EX60) showed multiple numbers of acellular plaques were detected in the mid brain, associated with oedema, hypoplasia, and congested blood capillary in the hippocampus.
(C) AD+MSCs (H&EX60) showed congestion in the blood vessels and focal gliosis in the cerebral cortex.
(D) AD+MSCs & inducer (H&EX60) showed congestion in the meninges, associated with focal hemorrhage and oedema with gliosis in the hippocampus.
(E) AD+MSCs & inhibitor (H&EX60) showed diffuse gliosis in the cerebral cortex, associated with focal hemorrhage in the brain stem.
(F) AD+ inducer (H&EX60) showed neuronal degeneration in the brain stem associated with focal gliosis in the cerebrum and congestion with hemorrhage in the hippocampus.
(G) AD+inhibitor (H&EX60) showed focal gliosis with plague formation observed in the cerebrum, associated with atrophy and gliosis in the hippocampus.
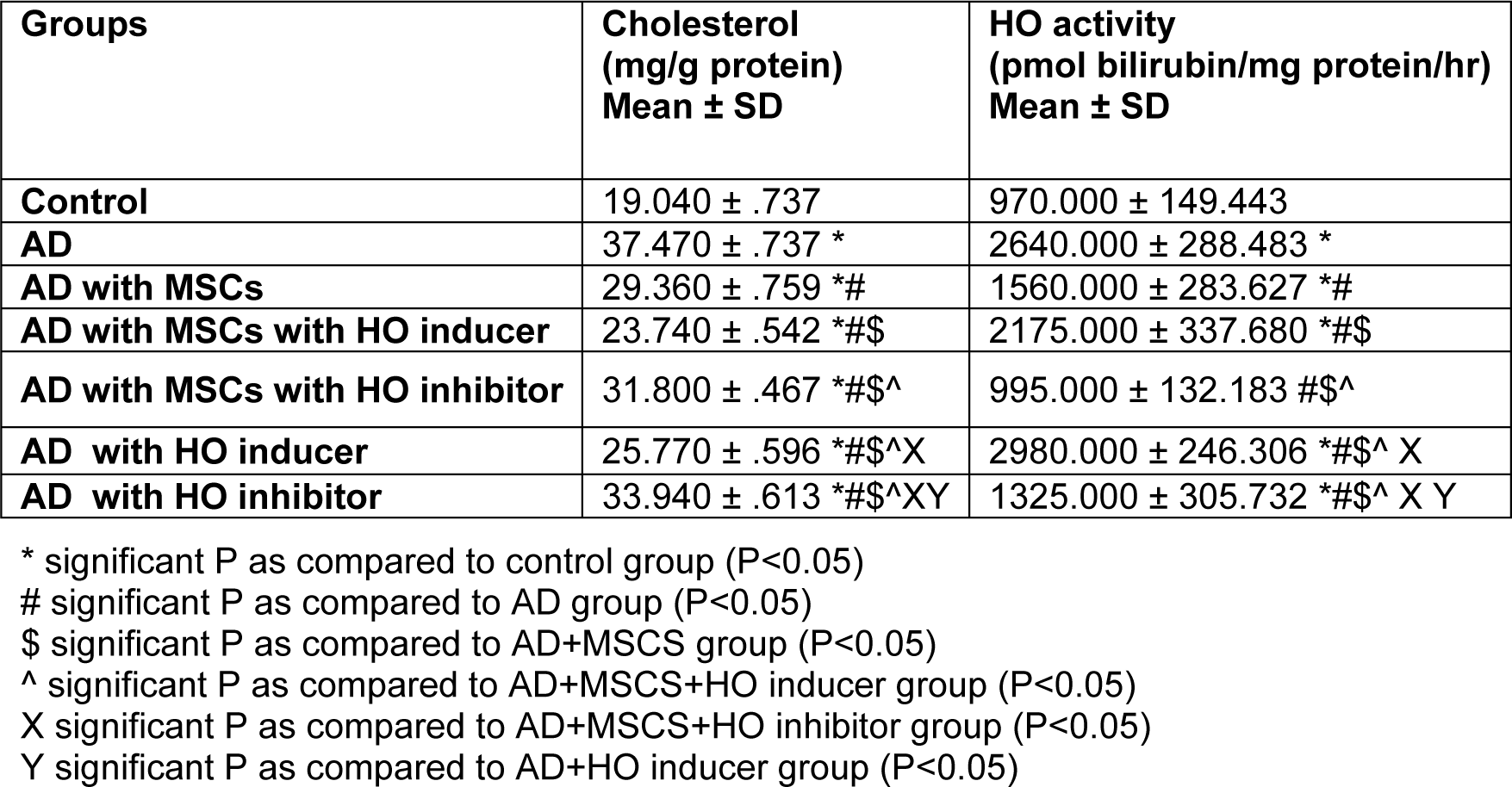
Table 2: Cholesterol (mg/g protein) & HO activity (pmol bilirubin/mg protein/hr) in different studied groups
[*] Corresponding Author:
Marwa Abdul latif Alkaffas, Department of Medical Biochemistry, Unit of Biochemistry and Molecular Biology, Faculty of Medicine, Cairo University, Cairo, Egypt; Mobile number: 0122206459, Fax number: 02023632297, eMail: Madho_taal@yahoo.com
