Research article
Nicotine exposure caused significant transgenerational heritable behavioral changes in Caenorhabditis elegans
Faten A Taki1, Xiaoping Pan1, Baohong Zhang1[*]
1Department of Biology, East Carolina University, Greenville, NC 27858EXCLI J 2013;12:Doc793
Abstract
Passive and active exposure to tobacco smoking among youth is directly associated with immediate as well as long-term health deterioration. Despite all public health policies and efforts, the percentage of teenage smokers is still relatively high, especially in developing countries. Very few, if any, studies have been done on the transgenerational effect of nicotine exposed during the more sensitive, early developmental stages. We employed C. elegans as a biological model to study the multigenerational impact of chronic nicotine exposure. Nicotine treatment was limited to N2 hermaphrodites of the F0 generation. Exposure was limited to the larval period L1-L4 (~31 hours) after which worms were transferred to a fresh NGM plate. N2 hermaphrodites at L4 developmental stage were used for behavioral analysis across three generations: F0, F1, and F2. Our results show that nicotine was associated with changes in sinusoidal locomotion, speed, and body bends in L4 larvae in all three tested generations. These behavioral alterations were not restricted to F0, but were observed in F1 and F2 generations which were never exposed to nicotine. Our study is the first to reveal that nicotine addiction is heritable using C. elegans as a model organism. These results underscored the sensitivity of early development stages, with hope to spread more awareness to encourage the avoidance of nicotine exposure, especially at a young age.
Keywords: nicotine, C. elegans, L4, post-embryonic stage, sensitivity to stress, transgenerational effect, addiction, behavior
Introduction
Efforts to set policies to optimize human health conditions have been continuously adopted to reduce and prevent diseases and health deterioration. With this in mind, serious research, programs and acts (e.g. TFI: tobacco free initiative) have been concerned with tobacco smoking-related diseases. Tobacco-smoking is responsible for the death of nearly 6 million individuals per year. Such represents half of the tobacco-exposed individuals, 10 % of which are second-hand smokers. The total percentage overrides deaths caused by the combined effects of HIV, alcohol, illegal drugs, murders, suicide, and vehicle-related injuries (CDC, 2008[4]). Also, based on WHO, second-hand smokers include about 40 % of children at home, a state of which doubles the likelihood of them growing up to be smokers. Unfortunately, tobacco negatively affects every organ. The consequential health deterioration (e.g. cardiovascular, respiratory and reproductive diseases, cancer) and premature death constitutes a major economic burden as productivity decreases (US DHHS, 2004[26]).
The addictive property of tobacco is attributed to nicotine (CDC, 1988[5], 2010[3]; Hilts, 1994[12]; Kandel et al., 1997[14]). Due to the complexity of studying such multi-faceted diseases, many scientists employed simpler organisms to dissect molecular as well as behavioral aspects of addiction (Matta et al., 2007[17]). In our study, we used C. elegans to investigate nicotine-associated behavioral effects. Research on C. elegans is free of ethical concern. The ease of maintenance, feasibility of biotechnological manipulations, and the conservation of major signaling pathways are a few of the advantages for choosing C. elegans as an established model organism in toxicogenomics research.
Nicotine-induced effects are complex and are mediated by several factors (e.g. environment, genetics) (CDC, 2010[3]). They are dose-dependent, but not monotonic. Nicotine caused a biphasic response where lower doses caused stimulation, while higher doses were associated with a depressant-like effect on the nervous system (Benowitz, 1988[1]). Regardless of the doses, nicotine is known to be a potent addictive drug of abuse. Several features are a prerequisite for addictive behavior and include symptoms of tolerance as well as withdrawal. Tolerance reflects desensitization and adaptation to the stressor (i.e. nicotine), in which the initial repetitive dose produces a lesser effect (e.g. ligand concentration at the receptor site). However, symptoms associated with drug abstinence are more reliable indices to study addiction (CDC, 2010[3]; Shiffman, 1989[21]). Taken together, the above points were taken into consideration in our experimental design.
In C. elegans, nicotine altered some behavior such as egg laying, pharyngeal pumping, muscle contraction, and male spicule ejection (Matta et al., 2007[17]). In addition, C. elegans was used as a model to study nicotine addiction. In fact, depending on the experimental design for nicotine treatment, the simple nematode responded with a set of complex actions and included an acute response, tolerance, withdrawal, and sensitization (Feng et al., 2006[9]). Unlike other models (e.g. rats), the half-life of nicotine in C. elegans is not known. Therefore, nicotine was constantly supplied during the exposure period.
Generally, a lot of research has been conducted on the toxicity and addictive properties and the prevalence of physical and psychological effects induced by nicotine (Benowitz, 1988[1]; Dani and Heinemann, 1996[7]; Feng et al., 2006[9]; Samaha et al., 2005[19]; Sobkowiak et al., 2011[23]). However, to our knowledge, the transgenerational effect of nicotine has not been well documented. There have been some reports about the heritability of smoking addiction (Heath et al., 1995[11]; True et al., 1997[25]); but the latter only studied the relationship between genes and the environment in smoking susceptibility. Research by NIDA and others (Kandel and Chen, 2000[14]; Slotkin, 2002[22]) reported that children and adolescents are much more sensitive to nicotine dependence than adults. Such sensitivity was reported within one generation, but very few research has been done to explore the trans-generational effect of drugs of abuse (Byrnes et al., 2012[2]). The greater extent of exposure (Kim et al., 2009[16]) and sensitivity in children stresses on the importance of evaluating the degree of effects not only on them as adults, but also on their off-springs. Therefore, the purpose of our study is to investigate the effect of nicotine across generations.
Materials and Methods
Nicotine exposure and sampling
Nicotine was purchased from (Acros Organics, NJ, USA). Nicotine was dissolved in phosphate buffer as 1 M and 0.001 M stocks. NaCl, peptone, agar and water mixture were first autoclaved and kept at 70 °C covered under the hood. Equal amounts were transferred to individual small autoclaved flasks cooled and kept at 55 °C. After the addition of cholesterol, CaCl2, MgSO4 and KH2PO4, nicotine solution was added to give the corresponding final concentrations 20 µm and 20 mM in the medium.
C. elegans hermaphrodite N2 Bristol wild type was used. Maintenance and worm transfer were done after NGM plates were seeded with OP50, and then kept at 20 °C. Egg synchronization was done via bleaching method described by Sulston and Hodgkin (1988[24]), with slight modifications. Briefly adult gravid worms were washed off the plate with M9 buffer into a 15 ml Falcon tube (for a medium sized pellet). Then the Falcon tube was centrifuged at 2000 rpm for 2 minutes to collect worm pellet and was followed by another wash. Then, 5 ml of synchronization solution was added for 5 minute-shake until the eggs were dispersed in solution. The eggs were pelleted after centrifugation at 2000 rpm for 2 minutes. The supernatant was removed and followed by four time wash using 5 ml M9 washes. The eggs were finally suspended in the last wash and were placed on a shaker in the 20 °C incubator for about 14 hours. After hatching, all progeny were stuck at L1. The latter were seeded onto corresponding treatment plates.
Figure 1(Fig. 1) shows the general protocol for worm treatment and sampling. F0-L1 larvae were transferred to the three treatment groups which included the control group along with the low and high nicotine concentrations. F0 exposure lasted around 31 hours until end of L3-beginning of L4. Later, around 20 worms were then picked from each of 4 replicates into 4-treatment matched 3.5 cm petri-plates. The plates were previously seeded with OP50 and left to dry and adapt for subsequent behavioral studies. Then, worms were washed off the plates and transferred to an Eppendorf tube.
Then, the pellet was washed twice with M9 interrupted by centrifugation and supernatant removal. The worms were then transferred into OP50-seeded NGM plates, left to dry. Afterwards, the plates were sealed and placed in the 20 °C incubator to grow until second day of adulthood-associated with egg laying peak. Plates were then washed for synchronization. The whole procedure was repeated twice until collecting the F2 generation.
Two hours after the transfer, a 5 minute-video was taken per replicate for every treatment group. The video was set at (15 frames/sec) with the same magnification for all treatment groups. The videos were then analyzed via Wormlab software (MBF Bioscience). Output data included endpoints for every tracked worm (i.e. mean track length, mean wavelength, mean amplitude, mean maximum amplitude, mean smoothed forward speed, mean smoothed backward speed, mean bending angle, omega bends, and reversals ratio). Video image noise was taken into consideration when choosing among the calculated speed indices. Image noise represents a signal detected during tracking, the former of which is generally not originating from the target (as in the case of uneven illumination). With the assumption that the target is associated with medium-sized features in comparison to the small-featured noise, a smoothing approach reduces the small features while preserving the larger shapes. Smoothing is applied to remove the unwanted variation (Kan, 2012[13]). So as others (Faumont et al., 2011[8]), the speed was calculated as the average instantaneous velocity over a specific time frame. With such rationale in mind, we chose smoothed speed to study the effect of nicotine on the locomotion velocity.
Data analysis
Data provided by Wormlab software was exported to an Excel sheet. Mean track length, wavelength, amplitude and maximum amplitude variable for each tracked worm were used from the track summary output. Each of the smoothed speed, bending angle, omega bend, and reversals ratios was calculated as the average/frame for each tracked worm. The smoothed speed was divided into positive (forward) and negative (backward) speeds. Both speeds were binned into intervals, and the number of worms with speeds falling in the right range was counted to get a frequency table.
Contingency tables were used for speed statistical analysis. The Chi-square test was used for overall statistical significance, and the speed pairwise comparison among treatment groups was based on the z-scores. As for the other endpoints, data from each individual worm was pooled from the four replicates per treatment group for statistical analysis via omnibus hypothesis testing one-way ANOVA. Statistical significance was reported when p < 0.05. Data analysis was done via SPSS (19). Each endpoint is defined based on Wormlab software as described in Figure 2(Fig. 2) (MBF Bioscience, 2012[18]).
Results
The transgenerational impact of nicotine on locomotion
In the F0 generation, the mean track length, amplitude, maximum amplitude, and wavelength, were significantly affected by direct nicotine exposure with F(2,460) = 29.655; F(2,460) = 52.635; F(2,460) = 150.104; and F(2,460) = 705.101 at P < 0.001. A peak was observed for the low concentration treatment groups for the track length and maximum amplitude values (P = 0.027). As the concentration increased, the values for all of the four endpoints significantly decreased (P < 0.001) when comparing the high concentration (20 mM) treatment groups to both control (0 µm) and low concentration (20 µm) treatment groups (Figure 3(Fig. 3)). In the following generations, for the most part, an increase was observed more noticeably in F1 after which it was weakened in F2. The effect on F1 showed statistically significance differences in track length [F(2,315) = 3.619, P = 0.028], maximum amplitude [F(2,315) = 3.715, amplitude [F(2,315) = 4.974, P = 0.007] and wavelength [F(2,315) = 7.206, P = 0.001]. Post-hoc pairwise comparison testing shows that the mean wavelength increased in both 20 µm (P = 0.001) and 20 mM (P = 0.002) treatment groups when compared to control. A dose-dependent increase in the amplitude and the maximum amplitude was observed to summit in the 20 mM treatment group (P ≤ 0.007). In fact, the track length and the amplitude were even noticeably greater than the 20 µm treatment group (P = 0.008 and P = 0.022, respectively). From F1 to F2, statistical significance was observed only in the wavelength [F(2,198) = 4.913, P = 0.016] where elevation was observed in both the low and high concentration treatment groups (P = 0.012 and P = 0.014, respectively). Interestingly, though not statistically significant, all of the locomotion endpoints were higher in the nicotine treatment groups than control (Figure 3(Fig. 3)).
Multigenerational effects of nicotine on the dynamic body movements on C. elegans
There was an opposing pattern between F0, and F1 and F2. In F0, the reversals decreased in a dose-dependent but not a statis-tically significant manner. However, both F1 and F2 increased in their reversals. The stronger increase was seen in F1 [F(2,236) = 3.939; P = 0.021]. The 20 mM treatment groups out-reversed the control (P = 0.008) and 20 µm treatment groups (P = 0.021). The same concentration continued was associated with more reversals than the lower nicotine concentration even at F2 (P = 0.045) (Figure 4(Fig. 4)).
A dose-dependent decrease in the average bending angle was observed in F0 and F2. With an impact factor of F = 39.336 at P < 0.001, F0 worms exposed to high nicotine concentration bent with a smaller angle than control and 20 µm treatment groups (P < 0.001). From F0 to F1, worms exposed to 20 mM nicotine continued to have a narrower bending angle than the 20 µm-exposed worms (P = 0.034) (Figure 4(Fig. 4)).
As for the omega bend, major differences were not observed in the 20 µm treatment group. On the contrary, an increase was evident in the 20 mM treatment group in all the generations. The increase was the strongest in F0 [F(2,460) = 10.039], particularly in the 20 mM treatment group when compared to both control and 20 µm treatment groups (P ≤ 0.001). The omega impact factor decreased in a generation-dependent manner to become F(2,315) = 4.375; P = 0.013 in F1. The increase was statistically significant with P = 0.023 and P = 0.004 when compared with control and 20 µm F1 groups, respectively. Despite the weakening of the effect trans-generationally, the dose-dependent increase was still observed in F2 with the 20 mM treatment compared to control with P = 0.027 (Figure 4(Fig. 4)).
The transgenerational effect of nicotine on speed on C. elegans
Forward speed
Nicotine exposure had the strongest impact on the F0 generation worm population on the overall forward speed among the treatment groups (χ2 = 68.707; P < 0.001). Contrary to the distribution of the worm proportion for the 20 µm treatment group which didn't deviate with statistical significance from the control at any speed range, the 20 mM worm proportion statistically differed from the control in 4 of the 5 speed ranges. Most of the 20 mM treated worms moved with forward speed falling in 0 -20 µm/s range. In the latter, our data reveal a ([52.3:47.7:84.5] %) relative worm distribution among for each of control, 20 µm, and 20 mM treatment groups, respectively.
Consequently, both the control and 20 µm treatment groups had a higher worm frequency in the speed ranges: 20-40 µm/s, 40-80 µm/s, 80-160 µm/s, > 160 µm/s. 14.3 % of the worms in the control, and 10.2 % of the worms in the 20 µm treatment group moved with speed range of 40-80 µm/s. Only 1.7 % of worms treated with the high nicotine concentration moved with that speed range. A similar pattern was observed at the > 160 µm/s speed range. An average of 3.5 % of the worms belonging to both control and the 20 µm treatment groups moved with forward speed > 160 µm/s, while only 0.4 % of worms belonging to the 20 mM nicotine treatment group moved at that speed range (Figure 5(Fig. 5)).
The impact on forward speed was robust as it was observed in the F1 generation (χ2 = 43.421; P < 0.001). The frequency of worms, exposed to 20 mM nicotine, continued to have a statistically significant peak ([19.8:12.4:53.6] %) in the 0-20 µm/s range. The relative worm peaks for those exposed to 20 µm nicotine showed a new set of proportions with statistically significant elevations ([19.8:24.7:8.9] %) and ([5.8:16.5:3.6] %) at speed ranges of 40-80 µm/s and 80-160 µm/s when compared to both control and 20 mM groups (Figure 5(Fig. 5)).
The worm proportions peaking at the same speed range became more similar in the F2 generation. However, the proportion of worms treated with 20 µm nicotine ([42.6:60.3:54.0] %) was statistically higher with respect to control at the 20-40 µm/s while both the control and the high concentration treatment groups had more worms with higher speed ranges e.g. ([16.4:9.5:22.0] %) at the 40-80 µm/s (Figure 5(Fig. 5)).
Backward speed
Nicotine exposure also significantly affected the worm's backward speed on F0 generation (P < 0.001). 76.6 % of the 20 mM treated worms belonged to the slowest speed range (0-20 µm/s) when only 23.8 % and 35.2 % of the control and 20 µm treated worms were in this range. Consequently, the proportion of the 20 mM treated worms decreased from 20.0 % to about 0.0-2.5 % for the subsequent faster speed ranges, three of which were statistically significant. Meanwhile, the difference in the peaks between control and treatment groups was statistically significant in the 20-40 µm/s speed range ([45.2:26.1:20.0] %). Also, though not significant, another high proportion of worms was observed for the 20 µm treatment group at the 40-80 µm/s ([23.8:30.6:25.5] %) (Figure 5(Fig. 5)).
Statistically significant differences in the worm proportions were also detected in the F1 generation and that was limited to the high concentration treatment group. 34.5 % of the 20 mM treated worms had a 0-20 µm/s speed range in comparison to the 18.6 % 20 µm treated worms. Another statistically significant difference was observed for the high-concentration treatment group (1.8 %) at the 80-160 µm/s speed range while the control and low concentration treatment groups had worm proportions of 10.7 and 14.4 %, respectively, at that range (Figure 5(Fig. 5)).
As for the F2 generation, though the worm proportion peaks became more alike and in the 20-40 µm/s range ([38.3:40.7:34.7] %), the 20 mM-nicotine-treated worms peaked with statistical significance at a faster range with 42.8 % of its worms at the 40-80 µm/s speed range while the 0 µm and 20 µm treatment groups had 25.0 % and 32.2 % of their worms in this speed range (Figure 5(Fig. 5)).
Discussion
Nicotine is a potent stimulant and a cholinergic agonist. There is no uniform standard molecular phenotype associated with nicotine as alterations in cholinergic receptors in the brain ranged from states like stimulation, inactivation of, and increase or decrease in the turnover rate of nicotinic receptors on the cell membranes. Its action is therefore not only context-dependent, but it is also based on the dose and duration of its exposure (Schafer, 2002[20]). In C. elegans, it has been documented that nicotine treatment is associated with hyper-contraction of body wall-muscles, stimulation of egg laying, increased pharyngeal pumping as well as a decrease in the efficiency of male spicule in mating (Matta et al., 2007[17]; Schafer, 2002[20]). Perhaps what is really interesting to us is the addictive nature of nicotine. With smoking being so prevalent in countries in the Middle East (e.g. Lebanon), the chances of persistent nicotine exposure among the younger groups remain high. Early developmental stages have been proven to be more sensitive to any sort of stresses. When considering nicotine, the case is not different. Of notice, the highest male to female-teenage smokers was reported in Lebanon, a 66:54 % in 2005-2010 (WHO, 2012[29]). It was reported that even a limited nicotine exposure during adolescence may lead to symptoms of dependence and that this sensitivity might be due to the neurochemical changes in the brain that is different from those of adults (CDC, 2010[3]; Slotkin, 2002[22]). We were interested in assessing the extent of the nicotine-induced alterations. We wanted to explore if effects caused by early development nicotine exposure would be passed on to the off-spring. Thus, nicotine exposure was limited to the L1-late L3-early L4 period. Hence, adult hermaphrodite worms and the subsequent F1 and F2 generations were never in direct contact with nicotine.
Understanding the patterns and relationships in our data
Speed can be calculated as wavelength x oscillation frequency. Therefore, the wavelength and the speed are directly proportional. That is consistent with our data as shown in the F0 generation; a decrease in speed in the 20 mM group was associated with a decrease in wavelength. Also, in F1 and F2 generations, both the forward speed and the wavelengths increased.
The omega bend is summarized in 3 steps: With reference to the body centroid point, the worm has a bending angle < 90o. Then, the worm bends to less than 45o. The omega bend ends with the worm opening its body with a bending angle > 90o. Hence, one would expect that there is an inverse relationship between omega bend and bending angle and such was observed in our results (Figure 4(Fig. 4)).
F0 generation models direct nicotine toxicity, and addiction (tolerance)
The high concentration treatment group modeled nicotine-induced toxicity as it was negatively affected in all the locomotive indices. Worms moved less as evident in the lower track length, and had lower wavelength and amplitudes. Also most of the worms had minimal forward and reverse speeds (0-20 µm/s). Thus the 20 mM treated worms seemed paralyzed, and that is in agreement with previously reported results (Sobkowiak et al. 2011[23]). Having said that, the increase in bends might not totally reflect the omega bends in specific. It seemed as if the worms were unable to free themselves and appeared to be in coiled structures (data not shown). The latter could have been mistakenly detected as omega bends by the software. The decrease in amplitudes in the 20 mM may support this conclusion as it is reasonable to expect a directly proportional relationship between omega bend and amplitudes.
Nicotine is involved in locomotion stimulation when applied acutely. The stimulating effect is evident when applied in a specific concentration range. The 20 µm treatment group falls within this range (Sobkowiak et al., 2011[23]). However, no increase in forward speed was detected. One difference in the experimental settings was the duration of nicotine application. Therefore, the “apparently” normal speed may represent chronic nicotine tolerance and adaptation which has been previously documented (Feng et al., 2006[9]). However, the worms did show a faster negative speed which reflects faster reversal movements. This is logical since the AVA command neurons, which regulate reversals, are nicotine targets (Chalfie et al., 1985[6]; Feng et al., 2006[9]; Von Stetina et al., 2006[27]; Zheng et al., 1999[31]). In normal food-replete conditions, worms tend to be “dwelling” - a behavior with frequent reversals and increased turn angles with lower forward speed. This was not totally observed in our case since the forward speed for the 20 µm group was not lowered. Instead, the worms performed fewer reversals and more omega bends.
The F1 and F2 generations modeled inherited toxicity and addiction (withdrawal)
The effect of nicotine on the forward speed
Overlaying the speed curves allowed us to see two major peaks in the F1 generation worm population. The control (0 µm) and 20 µm treatment groups had most of their worms moving in the 20-40 µm/s range, but with a general increase in speed among the 20 µm treated worms as 45.3 % of them were faster than those in the control (31.4 %). As for the 20 mM treatment group, the peak was in the 0-20 µm/s speed range. However, unlike the case in the F0 generation, we can notice the absence of any statistically significant difference in comparison to the 0 µm treatment group. Hence, in the F1 generation, more 20 mM-nicotine-treated worms moved with higher forward speed. Thus, their behavior is becoming closer to the wild type untreated worms.
Reaching the F2 generation, all of the treatment groups peaked at the same speed range (20-40 µm/s) with the 20 µm group having the largest worm proportion. On the other hand, taking into consideration the highest four speed ranges, it seemed that the highest worm proportion belonged to the 20 mM-treatment group (80 %), which is close to that of the 20 µm (79.4 %), and the least was that of the control (68.8 %) treatment groups.
The effect of nicotine on backward speed
We previously described a three-peak-speed-pattern in the F0 generation worm population occurring in the 20 µm treatment group which reversed faster than 0 µm and the 20 mM treatment groups, respectively. 77 % of worms treated with the 20 mM nicotine concentration moved at the 0-20 µm/s speed range, while only 34 % of their offspring in the F1 generation worm population moved at that speed. This proportion remained almost the same in the two successive faster speed ranges (33 %, 29 %, respectively). From a bird's eye view, it seems that two peaks appeared for F1. Most of the worms in the low and high nicotine treatment groups reversed with 40-80 µm/s speed, while those of the control group reversed with a slower speed (20-40 µm/s). Thus, the 20 mM treated worms became far from paralyzed, as was observed in the F0 generation, and seemed to be catching up with by increasing their reversing speed.
The pattern seems to get exacerbated in the F2 generation, as the proportion of worms treated with nicotine high concentration peaked at the faster speed 40-80 µm/s in comparison to both the control and the low concentration treated worms. The latter two shared their peak at the 20-40 µm/s.
Withdrawal serves as a better index than tolerance (CDC, 2010[3]). Both nicotine-dependent and nicotine non-dependent smokers did not differ in tolerance after being exposed to it. However, they differed significantly with their behavior during nicotine abstinence (CDC, 2010[3]). Interestingly, the phenotypes observed in the F1 and F2 generations may be models of withdrawal since worms were grown on fresh NGM all along, but still exhibited altered speed (Schafer, 2002[20]). The hyperactive behavior can be reflective of craving or uneasiness in worms as they are no longer getting their addicting and satisfying nicotine dose. Looking at our data, we suspect that this addicting dose (that is not associated with direct toxicity) is around that of the low concentration (20 µm range) as evident in the F1 individuals. It is expected that an effect might be diluted across generations. Progressing from the F1 to the F2, we observed attenuation of the 20 mM nicotine toxic paralyzing effect, until it became comparable to that induced by the 20 µm range. Eventually the grand-progeny of the 20 mM treated parents had the highest speed (most anxious) in the F2 generation. We can deduce that the higher the parental exposed concentration, the further down the effect is tracked and inherited.
Omega and reversals and overall locomotion indices in response to nicotine treatment
Three behavioral patterns are defined for C. elegans as a function of food supply. When food is present, short reversals and infrequent omega bends occur. When transferred to a food-free-medium, long reversals, frequent omega bends, and an increase in forward speed are observed. The third pattern is seen after longer periods of food abstinence, when both reversals and omega bends decrease to allow the worm to seek food. In short, the omega bends are generally proportional “coupled” to reversals-though the opposite is not a prerequisite (Gray et al., 2005[10]; Wakabayashi et al., 2004[28]). However, our data does not fully support this. In the F0 generation, the relationship between reversals and omega bend is opposite and this pattern was dose-dependent to become statistically significant at 20 mM treatment group. It is noteworthy to mention that this behavior was specific to the F0 individuals which were in direct nicotine exposure. The pattern was different in the F1 and F2 generations, both of which were not exposed to nicotine and modeled withdrawal. It is important to exclude any biased interpretations to nicotine specific, addiction independent, symptoms.
In the F1 and F2 generations, both the omega bends and reversals only differed in the high concentration treatment group. Though they were “coupled”, it still didn't model the normal situation where omega bends should have been infrequent due to the availability of food as seen in the control. Though the omega bends did increase in the three generations, the omega bends occurring in the F0 individuals had less amplitude than control and may therefore not be true omega bends, while those in the F1 and F2 individuals were more vigorous with increasing amplitude in reference with control. It is documented that when the environment is declining, the frequency of reversals and sharp turns increases, and vice versa (Gray et al., 2005[10]). Such may pinpoint that the worms were not comfortable in the normal settings, and perhaps they were in a “craving” status. The latter point can be complimented by the conclusion provided by Zhao et al. (2003[30]). They considered reversals as a way that allows the worm to constantly reassess its priorities (i.e. as reversals were initially a way of avoidance from harsh contact and later became a way of foraging). Hence, this shift in behavior is reflective of withdrawal symptoms and might insinuate the inheritance of nicotine addiction.
Logically, the alterations in reversals and body bends point to the effect of nicotine on particular neurons. It has been documented that the AVA neuron is involved in reversals, while the SMD, RIV, and SMB are involved in omega bends and regulation of its amplitude (Gray et al., 2005[10]). It would be interesting to dissect the cellular pathways involved in the response to nicotine. Whether it majorly involves acetylcholine receptors as upstream effectors or its acts directly on different effectors (e.g. serotonergic system) in a cell-type specific manner is worth further studying.
Acknowledgements
Dr. Baohong Zhang, Dr. Xiaoping Pan and Ms. Faten Taki declare no conflict of interest. This project is partially supported by Grant Number R03DA032515 from the National Institute on Drug Abuse. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Drug Abuse or the National Institutes of Health.
References
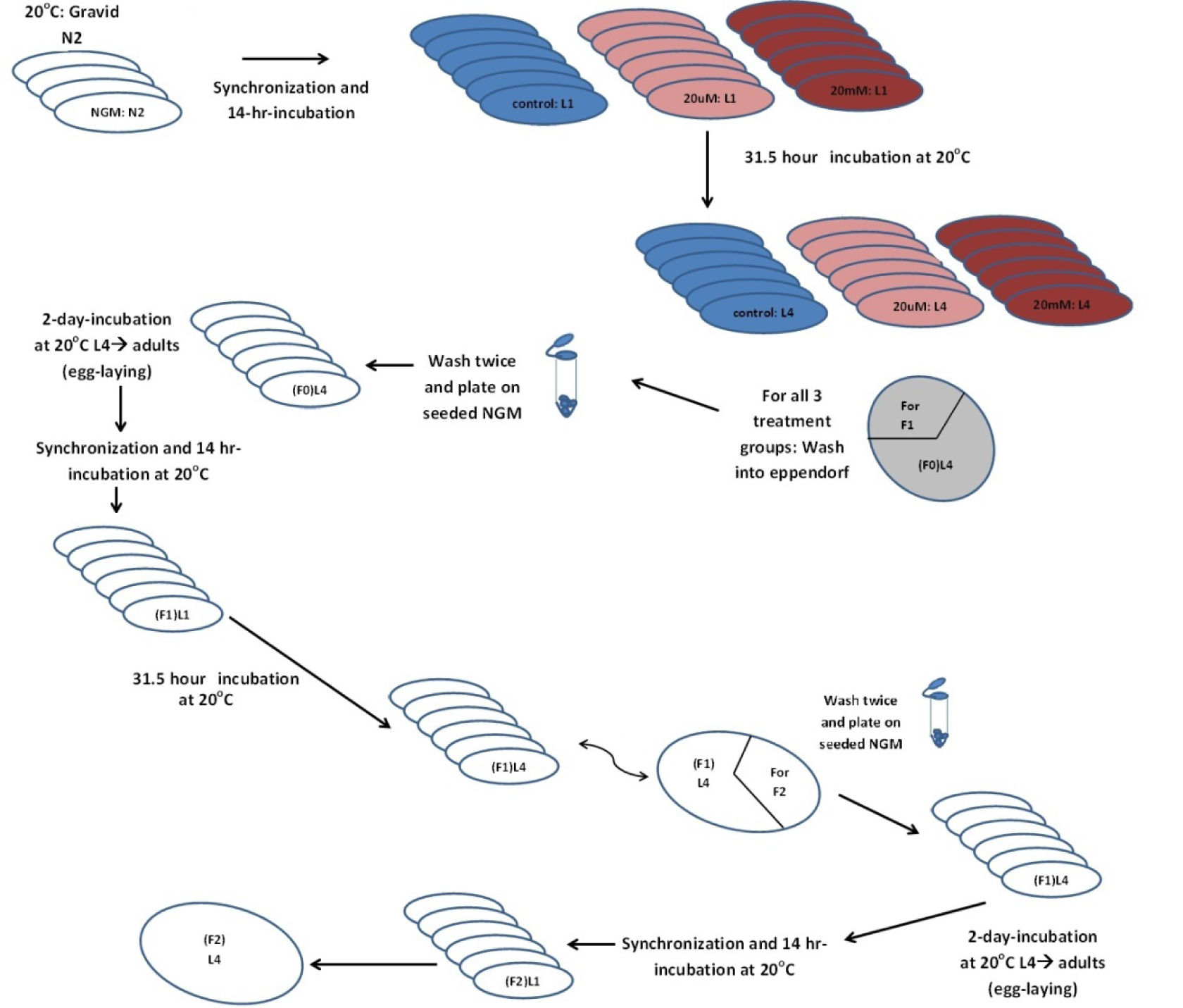
Figure 1: Description of nicotine exposure on C. elegans hermaphrodites and sampling for subsequent assays
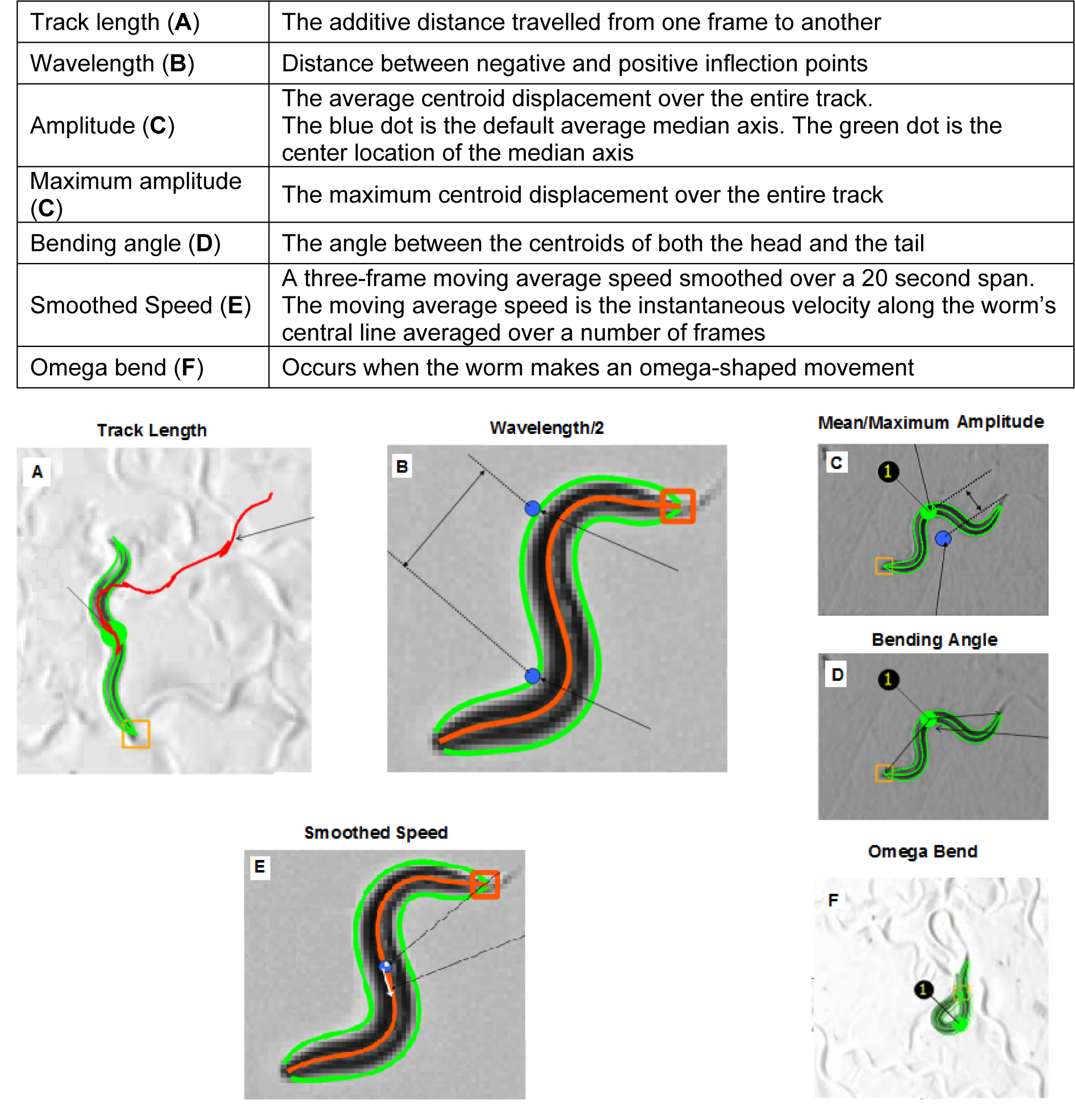
Figure 2: Summary of endpoint definitions as analyzed by the Wormlab MBF software. Letters on the top left of every picture correspond to the respective endpoints in the above table.
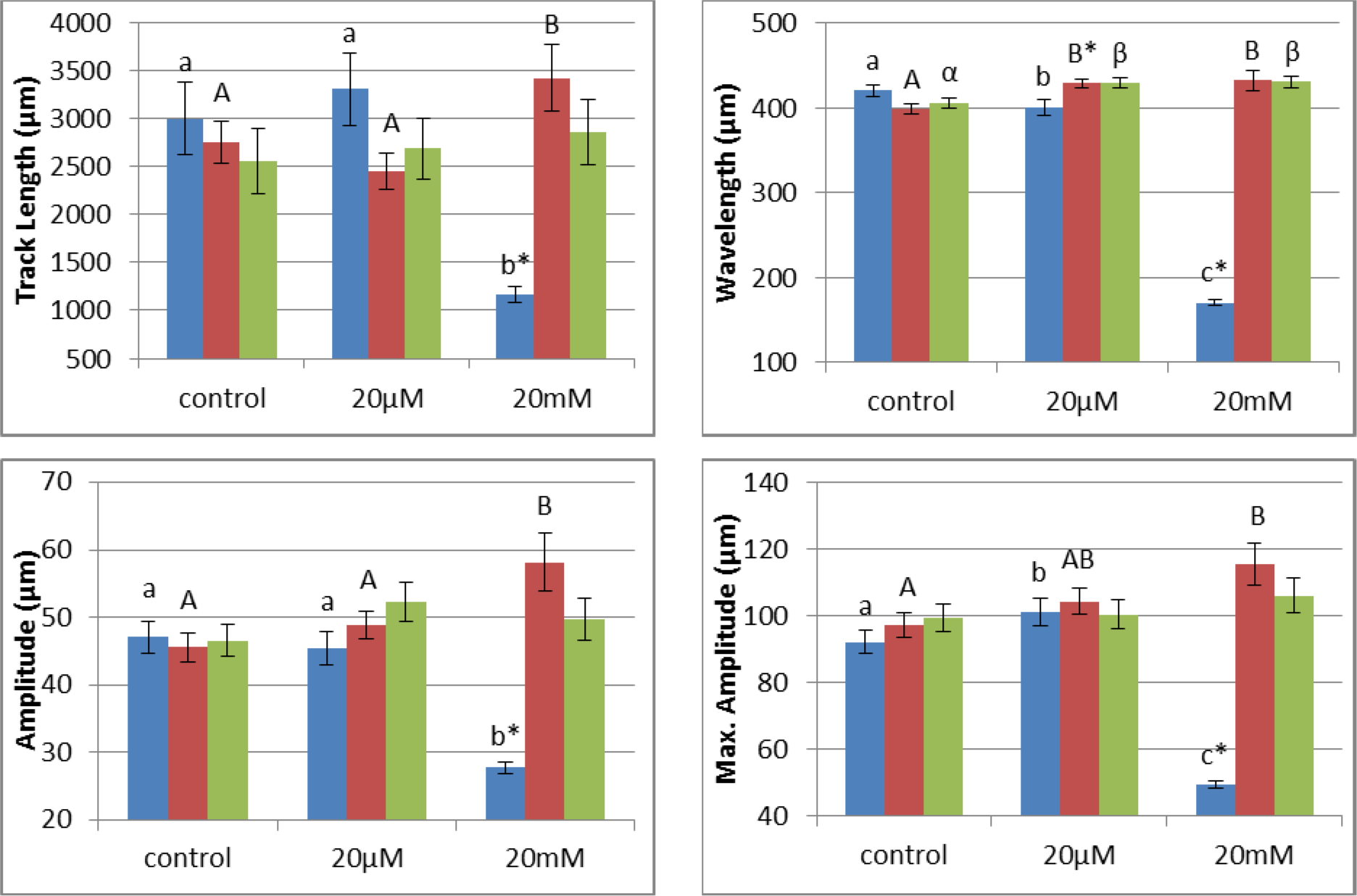
Figure 3: An overview of the variation in the different endpoints' pattern as a function of nicotine dose on L4 hermaphrodite C. elegans across the three generations. From left to right, bars represent F0, F1, and F2, respectively. The x-axis represents nicotine concentrations used. Control is the group without nicotine. (P* ≤ 0.001).
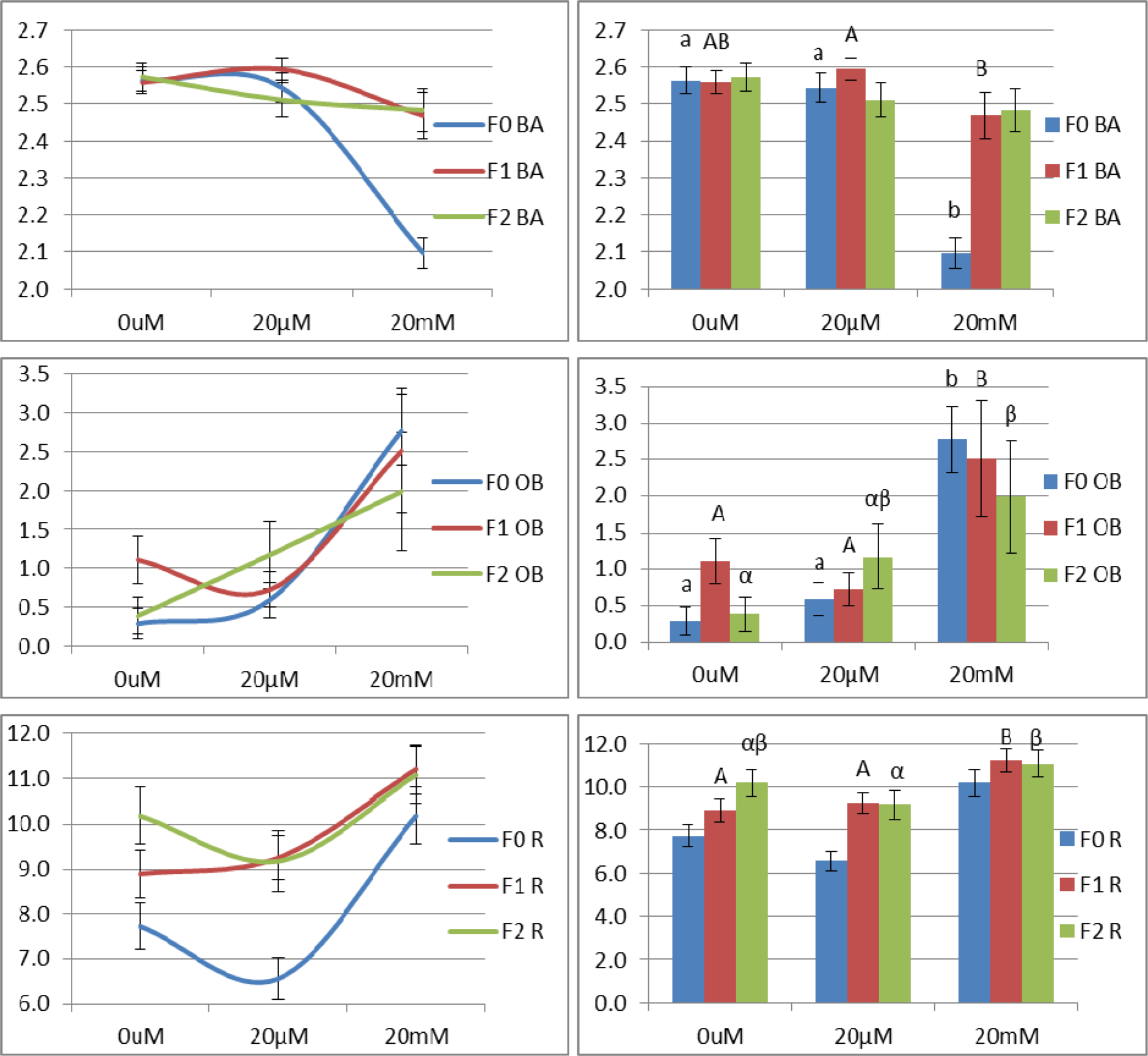
Figure 4: An overview of the variation in the patterns of body bends and reversal behavior in L4 hermaphrodite C. elegans as a function of nicotine dose across the three generations. BA: Bending angle; OB: Omega bend; R: Reversals. In the bar graphs, bars from left to right represent F0, F1, and F2 generations, respectively. Pairwise comparisons were performed among treatment groups within same generation. (ab), (AB), (αβ) are for F0, F1, and F2, respectively. Different letters correspond to statistically significant differences. (P*) ≤ 0.001.
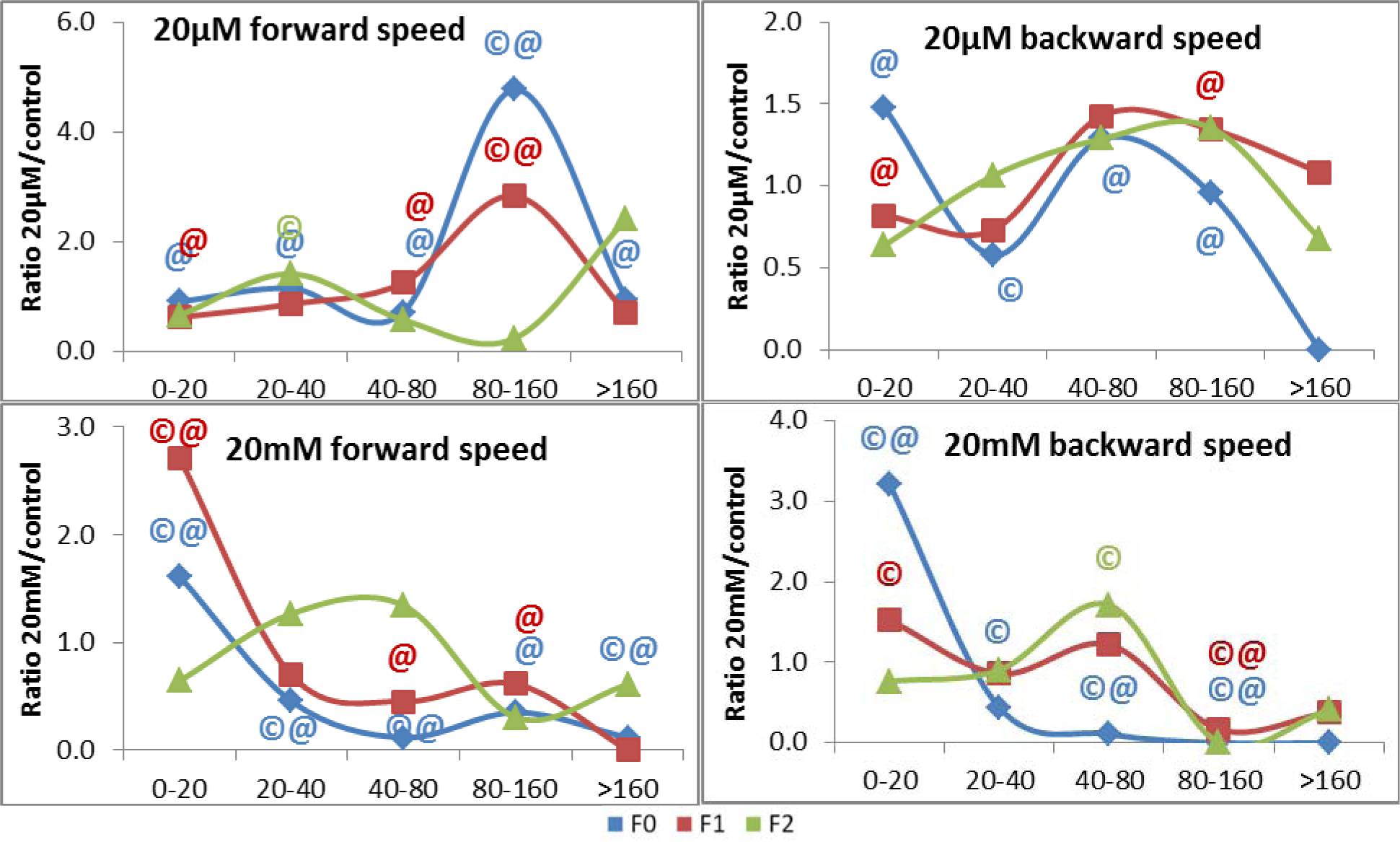
Figure 5: The impact of nicotine on the forward and backward speed (µm/s) in L4 C. elegans hermaphrodites. The y-axis represents a ratio calculated from the proportion of worms in each treatment group normalized to control. (©) represents p < 0.05 with respect to control. (@) represents p < 0.05 with respect to the other nicotine treatment group. It represents a ratio calculated from the proportion of worms in each treatment group normalized to control. The x-axis represents speed (µm/s) divided into 5 ranges.
[*] Corresponding Author:
Dr. Baohong Zhang, Department of Biology, East Carolina University, Greenville, NC 27858, USA, Tel : +1 252-328-2021, Fax : +1 252-328-4178, eMail: zhangb@ecu.edu