Research article
Chrysen-2-ol derivative from West Indian Wood Nettle Laportea aestuans (L.) Chew inhibits oxidation and microbial growth in vitro
Ganiyat K. Oloyede1[*], Martha S. Oyelola1
1Natural products/Medicinal Chemistry Unit, Department of Chemistry, University of Ibadan, NigeriaEXCLI J 2013;12:Doc894
Abstract
Bio-active compounds present in West Indian Wood Nettle Laportea aestuans (L.) Chew (Urticaceae), used in ethno medicine as antioxidant and antimicrobial were studied. The aim of this research work was to isolate and characterize the bio-active compounds in the n-hexane fraction of L. aestuans, determine the toxicity and subject it to in-vitro antimicrobial and free radical scavenging activities. The chemical constituents were isolated by gradient elution column chromatographic technique and Ultra Violet/visible (UV), Infrared (IR) and Nuclear Magnetic Resonance (NMR) spectroscopies were used for structural elucidation. The free radical scavenging activity of the isolate was assessed using three methods; scavenging effect on 2,2-diphenyl-1-picrylhydrazyl radical (DPPH), hydroxyl radical generated from hydrogen peroxide and ferric thiocynate method. Antimicrobial screening was done by agar well diffusion method while toxicity was determined by Brine shrimp lethality test.
Structures were proposed for the white crystalline solids isolated; (4E)-3,6-dimethylhep-4-en-3-ol (AB) and 1,2,3,4,4a,4b,5,6,6a,7,8,9,10,10a,10b,11-hexadecahydro-1,1,6a,10b-tetramethyl-7-((E)-4,7-dimethyloct-5-enyl) chrysen-2-ol (AC). Percentage yield of AC was 91.2 and was non-toxic with LC50 (µg/ml) value of 1581233000.0. AC significantly scavenged free radical at 0.0625 mg/ml in the DPPH (64.73 %) and hydrogen peroxide (99.22 %) tests. It also showed 65.23 % inhibition at 1.0 mg/ml in the ferric thiocyanate test. AC also inhibited microbial growth significantly when compared with gentamicin and tioconazole which are antibacterial and antifungal standards respectively. The presence of Chrysen-2-ol derivative in L. aestuans which was non-toxic and possessed significant antimicrobial and antioxidant activities supports its ethno medicinal application.
Keywords: Laportea aestuans, (4E)-3,6-dimethylhep-4-en-3-ol, chrysen-2-ol, toxicity, antimicrobial, antioxidant
Introduction
The potential of plants as a veritable source for pharmaceuticals and other therapeutic materials have been emphasized. Plants with medicinal values often referred to as secondary metabolites have been documented in herbal pharmacopeias, directories and books (Gbile, 1986[16]; Ghani, 1986[17]; Dokosi, 1998[12]). Secondary plant metabolites also referred to as natural products are renowned for their potent pharmacological activities; as antimicrobial, insecticidal, anticancer, antioxidant, pesticidal, amongst others. Some are odoriferous and find application in perfumes and cosmetics, for flavoring foods and drinks and for screening incense and household cleaning products. They include alkaloids, flavonoids, steroids, coumarins, anthocyanins, terpenoids and essential oils (Brinker, 1998[6]; Burkill, 1985[7]; Gbile, 1986[16]; Abu-Rabia, 2005[1]; Gilbert and Martin, 2006[18]). In recent years, there has been an extensive growth in natural product chemistry. This is due to advances in isolation, characterization, identification, purification and synthesis of new natural products. Many of today's medicines are obtained directly from natural sources and a renewed interest in the screening of plants for biological activity is due to the developments of in vivo and biochemical screening methods (Kokwaro, 1993[24]; Heywood, 1993[21]).
Laportea aestuans, the West Indian wood nettle, is an annual herb of the Urticaceae or nestle family. It is native to tropical Africa, although it is now widespread as an introduced species throughout both the western hemisphere and eastern hemisphere tropics and subtropics, including the USA. Laportea aestuans (Linn) Chew have the following synonyms; Urtica aestuans, Fleurya aestuans (Gaud) and Fleurya aestuans (Linn.) Miq. It is used as abortifacient, antimicrobial, laxative, in eye treatments and pain-killers. It is also used in treating pulmonary and stomach troubles, diarrhea and dysentery (Chew, 1969[9]; Burkill, 1985[7]; Friis, 1993[15]; Nadine, 2001[31]). Laportea aestuans (L.) Chew is recorded as an ethno veterinary species that is used for urinary problems of ruminants in Trinidad and Tobago and also to shorten labour and remove the placenta during childbirth (Lans, 2006[26], 2007[25]). Epidemiological studies indicated that consumption of L. aestuans inhibits the damaging activities of free radicals in human body (Morrison and Twumasi, 2010[29]). Free radicals find their way into the human body via metabolic pathways within body tissues and also from external sources such as food, drugs and pollution from the environment (Miller and Britigan, 1997[28]). It was also suggested that free radicals, such as superoxide radical (O2-) and hydroxyl radical (OH-) and non-free radical species, such as hydrogen peroxide (H2O2) and singlet oxygen (1O2) within the human body facilitate cellular injury, ageing, development of neurodegenerative and cardiovascular diseases (Stadtman, 1992[42]; Ames et al., 1993[3]; Alan and Miller, 1996[2]; Knight, 1998[23]; Pinzino et al., 1999[37]; Cadenas and Davies, 2000[8]; de la Fuente, 2000[10]; Stanner et al., 2004[43]). Consumption of fruits and vegetables has proven to substantially reduce the risk of cardiovascular diseases and neurodegenerative diseases, including Parkinson's and Alzheimer's diseases (Di Matteo and Esposito, 2003[11]; Bjelakovic et al., 2007[5]). Paul (2008[36]) isolated a mixture of methyl esters of aliphatic acids from Laportea aestuans. Also, the essential oil from the plant was found to be dominated by methyl salicylate and had significant antioxidant and antimicrobial activities (Oloyede, 2011[32]) while phytochemical, toxicity, antimicrobial and antioxidant screening of extracts obtained from Laportea aestuans (Gaud) had also been investigated (Oloyede and Ayanbadejo, 2013[33]). Based on our findings, there was need to isolate and characterize the bio-active compounds in Laportea aestuans. The prevalence of microbial infection and oxidation reaction is also a major driving force for this study. This current study is therefore aimed at isolating and characterizing the plant's secondary metabolite using chromatographic and spectroscopic techniques (UV, IR and NMR). Antioxidant and antimicrobial screening of the isolated pure compound was also carried out and the toxicity level was determined using Brine shrimp larvae eggs.
Materials and Methods
Chemicals and reagents
Dimethylsulphoxide (M&B, England), hydrogen peroxide (Merck, Germany) and 2,2-diphenyl-1-picrylhydrazyl (DPPH), silica gel 60-200 mesh size, ascorbic acid, butylated hydroxylanisole (BHA), rutin and α-tocopherol were obtained from Sigma Chemical Co (St Louis, MO). Brine shrimp larvae eggs were obtained from Ocean Star International, Inc. Company, USA. Acetone, chloroform, ethyl acetate, n-hexane, methanol, butanol, chloroform, hydrochloric acid, ammonia solution, naphthol, bismuth nitrate, potassium iodide, sodium hydroxide, copper acetate, NaOH, ferrous chloride, sodium chloride, copper sulphate pentahydrate, ferric chloride, conc. tetraoxosulphate (VI) acid, conc. HCl, ammonia solution, sodium potassium tartarate, linoleic acid, ammonium thiocynate, ethanol, hydrochloric acid, potassium chloride, glacial acetic acid, disodium hydrogen phosphate and dihydrogen potassium phosphate were all BDH general purpose chemicals and solvents were distilled prior to use.
Equipment and apparatus
Rotavapor RII0 (Buchi, England), Soxhlet apparatus, Ohaus analytical balance (USA), Steam Bath (Gallenkamp), silica gel GF254 (precoated aluminium sheets - Merck Germany), pH meter (Jenway model), Gallenkamp melting point apparatus, UV-Visible spectrophotometer (UVD-2960 model equipped with a UVWIN software version LABOMED INC, USA). IR Spectrophotometer (Perkin Elmer FT-IR system, Spectrum BX model of IR Spectrophotometer equipped with a PC and a Printer, England), Varian-Mercury NMR Spectrophotometer operating at 200 MHz for H and 50 MHz for C nuclei.
Plant collection and identification
Fresh leaves of L. aestuans weighing 13.4 kg were collected from the Botanical Garden, University of Ibadan, Nigeria. Identification of sample was done at the herbarium of Botany Department of the same University in January, 2011, by Mr. Owolabi and at the Forestry Research Institute of Nigeria (FRIN) by Mr. Afilaka.
Test organisms
Escherichia coli, Staphylococcus aureus, Bacillus subtilis, Pseudomonas aeruginosa, Klebsiellae pneumonae, Salmonellae typhi, Candida albicans, Aspergillus flavus and Fusarium moniliformes (Microorganisms were collected from the stock of the Department of Microbiology, Faculty of Science of University of Ibadan). The test organisms were maintained on nutrient agar slopes and kept in a refrigerator at 4 °C. Aliquots of nutrient broth (100 ml) were inoculated with the culture of test micro-organisms using a loop and then incubated at 37 °C for 24 h.
Reference standards
Gentamicin (80 μg/ml) and nystatin (80 μg/ml) were standards for bacteria and fungi respectively for the antimicrobial screening. Ascorbic acid, butylated hydroxyanisole (BHA), α-tocopherol and rutin were used for antioxidant activity. Dimethylsulfoxide (DMSO) was used for cytotoxicity studies.
Sample preparation
Whole plant of L. aestuans was collected, sorted to remove dirt, weighed and air dried for 3 weeks until the weight was constant and then pulverized using mill machine at the Mechanical Department of the Faculty Engineering of the University of Ibadan. The pulverized sample (2.355 kg) was kept for further analysis.
Extraction / purification procedure
Hot extraction was carried out by the use of improvised Soxhlet apparatus. The ground plant sample (2.35 kg) was packed and 5 Lt hexane was poured over the packed sample in the aspirator bottle. The extraction process continued until a colorless or clear hexane was observed indicating the end of the extraction. The extracts were collected and concentrated with the aid of a Bucchi rotavapor and stored in a desiccator prior to further analysis.The percentage yield of the extract was determined by the percent recovery technique. Thin Layer Chromatography (TLC) was employed using silica gel 60 F254 precoated plates and solvent system: hexane: ethyl acetate (2:5), (2:7), (3:1), (4:1), (5:1), (6:1), (10:1), (15:1). TLC was carried out on the crude extract to estimate the number of components. One drop of glacial acetic acid was added to each solvent system to aid distinct separation of spots into their different components. After the development, the chromatogram was then allowed to dry before it was transferred into the iodine tank. Initial spotting of the crude extract obtained on TLC plates indicated more than one components, hence the need for proper separation with a column chromatograph. Liquid column chromatograph used to separate non-volatile or thermally stable compounds was used to separate the components of the crude extract. 3 cm x 100 cm column size and a silica gel of mesh size 60-200 were used. Silica gel (180 g) was made into slurry by adding the solid adsorbent to a quantity of packing solvent (hexane). The slurry was swirled until it was homogeneous and relatively free of entrapped air. Prepared slurry was slowly poured into the column with the tap opened such that it gently settled uniformly. The column was constantly tapped to get rid of any trapped air bubbles and also ensure a leveled layer of the adsorbent. 6 g of crude extract was pre-adsorbed on silica gel. Hexane was used to wash the packed column prior to analysis. This pre-adsorbed sample was allowed to dry before loading onto the column. The ratio of the silica gel to that of extract was 30:1. The loaded sample was then covered with pure white sand and a small piece of cotton wool. The top of the column was usually filled with enough solvent/mixture of solvents to avoid cracking. The elution started with 100 % non-polar hexane after which the polarity of the solvent(s) was increased gradually with EtOAc until 100 % in a gradient elution chromatographic technique. The solvent system used in eluting the column was as follows: hexane 100 %, ethyl acetate/hexane 5 %, 10 %, 15 %, 20 %, 25 %, 40 %, 50 %, ethyl acetate 100 %. A total of 143 eluents of 100 ml each were collected. TLC of the eluents collected was also carried out using the same solvent system employed in the column chromatography. This was done to know the purity of each eluent, ascertain the number of components in it and also to pool together similar eluents which contain the same component based on their Rf values. Fractions 38 and 39 and 53 to 60 which were solids obtained from column chromatography were dissolved in hot ethyl acetate and hot acetone respectively and allowed to cool in ice bath in order to form precipitated solutions. The precipitates formed on cooling were separately filtered by vacuum filtration. The crystals were tagged AB and AC. The crystals were left at room temperature for three days in order to dry them. Melting point of pure compounds was determined with a Gallenkamp melting point apparatus. Spectroscopic analysis, UV-Visible, Infra-red and NMR spectrometry was used to characterize the compounds. The UV/Visible Spectra of 0.01 % w/v of the fractions were determined with the aid of UV-Visible spectrophotometer (UVD-2960 model equipped with a UVWIN software version LABOMED Inc., USA). Vmax (cm-1) from IR data also confirmed the structures. The Infrared spectrum of the pure compound was determined using a Perkin Elmer FT - IR Spectrophotometer, England). The KBr disc method was used for the preparation of the sample. The relative strength and position of all absorption in the infrared region was determined. The 1H and 13C NMR spectra of the pure compounds were determined using a 200 MHz machine for 10 % (w/v) solutions in deuterochloroform. Pulse irradiation technique employed was FT NMR at ambient temperature. Solid AC with higher yield and promising antioxidant activity in the preliminary screening was investigated for antioxidant and antimicrobial activity and its toxicity was also determined
Antioxidant activities of AC
Scavenging effect on DPPH
2, 2-diphenyl-1-picryhydrazyl radical (DPPH), a stable radical decolorizes in the presence of a free radical scavenger and decreases in absorption in a UV/Visible spectrophotometer when measured 10 min later against that of the control at 517 nm. Antioxidant activity of solid AC and antioxidant standards butylated hydroxylanisole (BHA), rutin, α-tocopherol and ascorbic acid were determined using the DPPH free-radical scavenging method. A 100 µm solution of DPPH was prepared by dissolving 3.94 mg in 100 ml methanol. To 3.0 ml of the methanol solution of DPPH was added 0.5 ml of the samples with doses ranging from 1.0 - 0.0625 mg/ml. Average results from triplicate analysis were recorded and the percentage inhibition of DPPH discoloration was calculated as % inhibition = {(ADPPH - AS)/ADPPH} x 100. Where AS is the absorbance of the sample solution and ADPPH is the absorbance of the DPPH solution (Hatano et al, 1988[20]; Gulcin et al, 2002[19], Pourmorad, 2006[38]; Mutee, 2010[30]).
Scavenging effect on hydrogen peroxide
Solid AC and standards butylated hydroxyanisole (BHA), ascorbic acid and α-tocopherol at the following concentrations; 1.0 - 0.0625 mg/ml were each added to a solution of 2 mM hydrogen peroxide, prepared in phosphate buffered-saline (PBS) pH 7.4. Decrease in absorbance of H2O2 was determined in a UV/Visible spectrophotometer at 285 nm after 10 min against a blank solution containing the sample in PBS without H2O2. Average result from triplicates analysis was recorded and was used to calculate percentage inhibition (Sies, 1997[40]; Soares et al., 1997[41], Oloyede and Farombi, 2010[34]).
Antioxidant activity by ferric thiocynate method
Ferric thiocynate method which is another antioxidant screening technique was used to screen compound AC obtained from L. aestuens and standards for antioxidant activity. Various concentrations (1.0 - 0.0625 mg/ml) of sample were prepared from a stock solution containing 10 mg of crystal in 99.5 % of ethanol. A mixture of 4 ml of 0.05 M phosphate buffer (pH 7.0), water (2 ml), sample (2 ml) and 2.0 ml of 2.51 % linoleic acid in 99.5 % ethanol were placed in a vial with a screw cap and placed in the dark in an oven at 6 °C. To 0.1 ml of this sample solution was added 10 ml of 75 % ethanol, 0.1 ml of 30 % ammonium thiocynate and 0.1 ml of 2 x 10-2 M ferrous chloride in 3.5 % hydrochloric acid, the absorbance of the red color which developed was measured at 500 nm after 3 min. Standards were also subjected to the same experiment. Average result from triplicates analysis was recorded and inhibition of lipid peroxidation in percentage was calculated from this equation:
% Inhibition = 1 - (A1/A2) X 100.
Where A1 was the absorbance of the sample and A2 was the absorbance of control reaction (Oloyede et al., 2010[35]).
Toxicity analysis
Brine shrimp lethality test
The toxicity of compound AC to living organisms was predicted using the Brine shrimp lethality test (BST). The shrimp's eggs were hatched in sea water for 48 h at room temperature. The nauplii (harvested shrimps) were attracted to one side of the vials with a light source. Solutions of the sample were made in DMSO, at varying concentrations (1000, 100 and 10 µg/ml) and incubated in triplicate vials with the brine shrimp larvae. Ten brine shrimp larvae were placed in each of the triplicate vials. Control brine shrimp larvae were placed in a mixture of sea water and DMSO only. The vials were examined against a lighted background after 24 h and the average number of larvae that survived in each vial was determined. The concentration at fifty percent mortality of the larvae (LC50) was determined using the Finney computer programme (Meyer et al., 1982[27]; Falope et al., 1993[14]; Keddy et al., 1995[22]).
Antimicrobial screening
Agar diffusion: pour plate for bacteria
The following organisms; Escherichia coli, Staphylococcus aureus, Bacillus subtilis, Pseudomonas aeruginosa, Klebsiellae pneumonae and Salmonellae typhi were used. Preparation of culture was done overnight and 0.1 ml of each of the organism was taken into 9.9 ml of Sterile Distilled Water (SDW) to give 10 ml of 1:100 (102) dilution. A 0.2 ml of 1:100 dilutions was taken into the prepared molten Nutrient Agar (NA) at 45 °C and was aseptically poured into the sterile plates and allowed to set on the bench for 45 min. A sterile cork-borer was used to create wells inside the set plates and different prepared concentrations of the sample as well as the positive and negative controls were introduced using syringes. The positive control for bacteria was gentamicin (80 μg/ml). The plates were left on the laboratory bench for two h before incubation at 37 °C for 24 h. Clear zones of inhibition were observed and the diameters were measured in millimeter (mm) using a transparent well-calibrated ruler. The average of triplicate readings were calculated and recorded (Schmidt, 2006[39]; Duraipandiyan et al., 2006[13]).
Agar diffusion: surface plate for fungi
Candida albicans, Aspergillus flavus and Fusarium moniliformes were the fungal strains used in this experiment. Molten sterile Sabouraud Dextrose Agar (SDA) was poured aseptically into the sterile plates and allowed to cool for 45 min. A 0.2 ml of 1:100 dilution of the organism was spread on the surface using a sterile spreader and a sterile cork-borer was used to create wells inside the plates. The same procedure described for anti-bacterial activity above was followed from this stage. The positive control for the fungi was tioconazole (80 μg/ml). All the plates for the fungi were incubated at 28 °C for 48 h. The clear zones of inhibition were observed and recorded using the same method as described for bacteria (Bayer et al., 1986[4]).
Data analysis
The mean ± SEM was calculated for each parameter and was analyzed separately using ANOVA followed by Dunnets 't' test. Toxicity results were analyzed using the Finney computer programme.
Results
Thin Layer Chromatographic analysis (TLC) of the crude extract gave five components with Rf values 0.39, 0.46, 0.69, 0.84 and 0.94. Column chromatography gave 143 fractions and were bulked based on similarity of their Rf values as A1, (fractions 1-20), AA (fractions 21-25), A2 (Fractions 26-37), AB (fractions 38 and 39), A3 (fractions 40-52) and AC (fractions 53-60). The remaining fractions 61-76, 77-78, 79-81, 82-89, 90-106, 107-118, 119-125, 126-128, 129-133, 134-140, 141-143 were also pooled accordingly. AA, A1, A2 and A3 were oily substances while AB and AC were crystalline solids. They all gave single spot in TLC analysis. Fraction AB (yield; 77.7 %) and AC (yield; 91.2 %) were further purified by crystallization. Fraction AB which was light brown solid dissolved in hot ethyl acetate and was allowed to cool in ice bath. The precipitate formed was filtered off giving white solid substance. Isolate AC, a greenish needle - like crystalline solid was dissolved in hot acetone and allowed to cool in ice bath. The precipitate formed was filtered and a white crystalline solid was obtained. Gallenkamp melting point apparatus was used to determine the melting point of the solids. Melting points of AB and AC were 76 °C-78 °C and 131 °C-132 °C respectively. Structures were proposed for solids AB and AC based on the spectra data obtained from UV/visible, infrared (IR), Proton and Carbon 13 Nuclear Magnetic Resonance (1H and 13C NMR) spectroscopies and were in complete agreement with literature values.
Structures of the compounds reported here are as shown in Figure 1(Fig. 1).
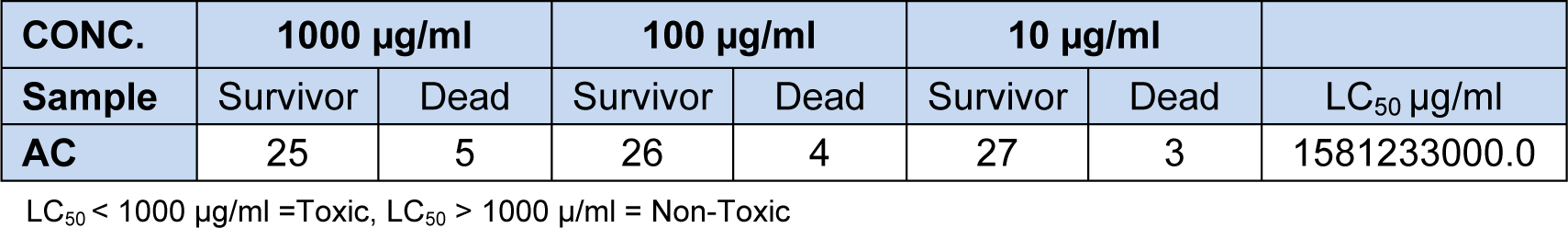
Brine shrimp toxicity test
AC was subjected to brine shrimp toxicity test and LC50 of 1581233000.0 μg/ml was obtained indicating non-toxicity (Table 1(Tab. 1)).
Antioxidant activity
Scavenging effect on DPPH
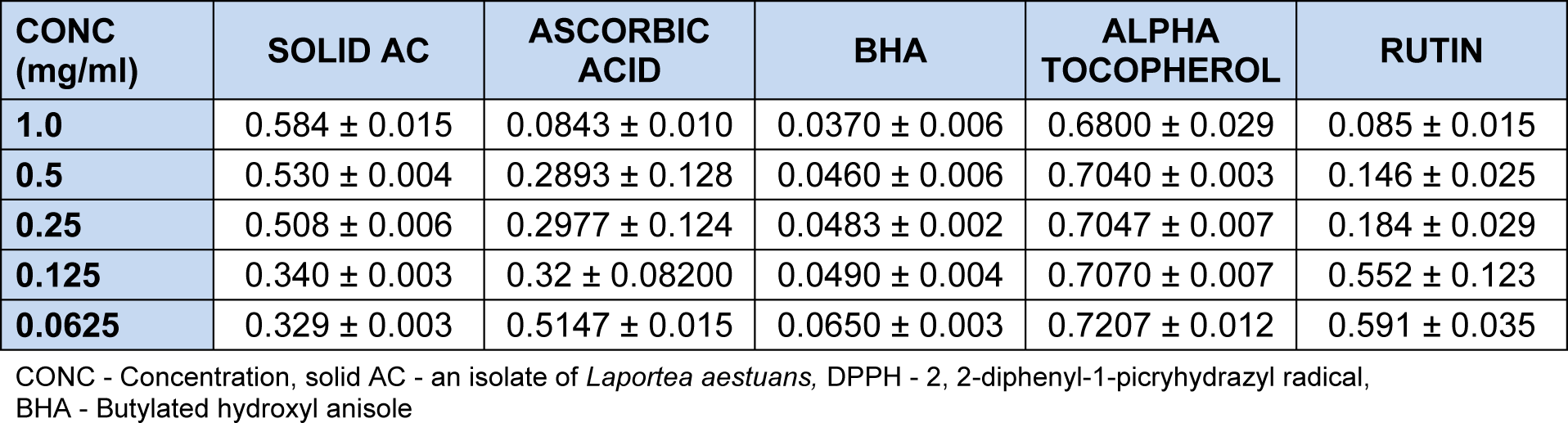
The reduction in absorbance of DPPH at 517 nm caused by the Solid AC was measured in triplicate after 10 min. Absorbance values decreases as the concentration decreases (Table 2(Tab. 2)). Solid AC was significantly active as a free radical scavenger at 0.0625 mg/ml (64.73 %) when compared with controls, ascorbic acid, rutin and α-tocopherol but lower than the activity of butylated hydroxylanisole (BHA). The percentage inhibition increases as the concentration decreases unlike in the controls. DPPH is known to be a stable free radical and accepts an electron or hydrogen radical to become a stable diamagnetic molecule.
Scavenging effects on hydrogen peroxide (H2O2)
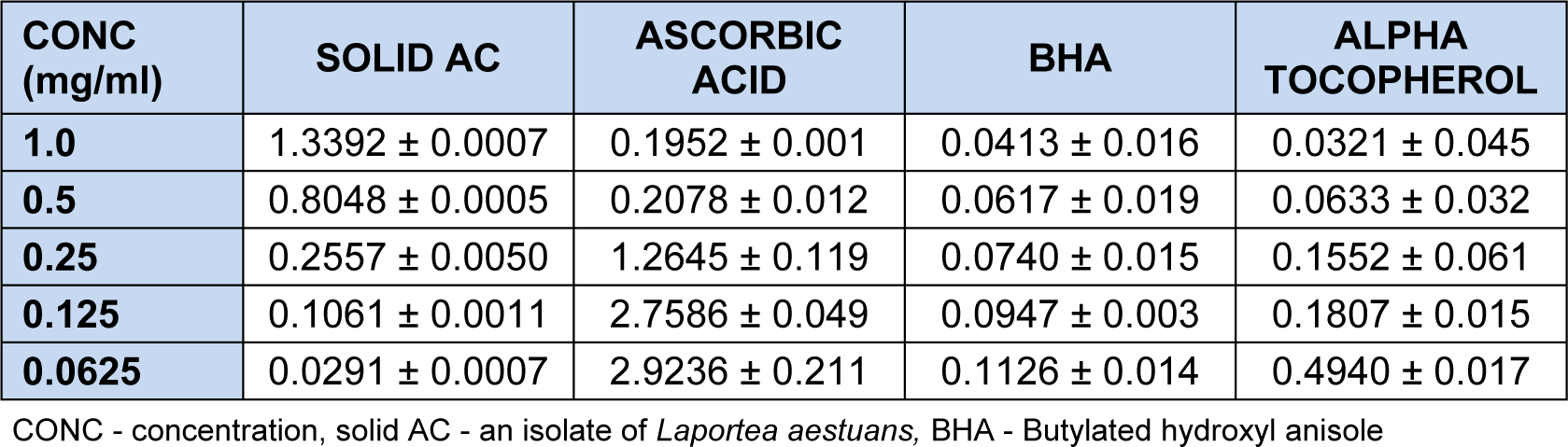
Absorbance measurement in the scavenging activity of solid AC and antioxidants standards, ascorbic acid, butylated hydroxyanisole (BHA) and α-tocopherol on H2O2 is shown in Table 3(Tab. 3). Scavenging effect on H2O2 was measured in triplicates after 10 min of incubation at 285 nm. At concentration of 1.0 - 0.065 mg/ml, AC scavenged hydroxyl radical in a concentration dependent manner better than the standards. Absorbance values decreases as the concentration decreases (Table 3(Tab. 3)), indicating better activity. Hydroxyl radicals are usually involved in radical chain or oxidation reactions which cause diseases such as cancer, nuero-degeneration, Parkinson's disease amongst others. At 0.0625 mg/ml, AC had 99.22 % inhibition which is better than that of ascorbic acid (22.43 %), butylated hydroxyanisole (BHA) (97.0 %), and α-tocopherol (86.9 %).
Antioxidant activity by Ferric thiocyanate method (FTC)
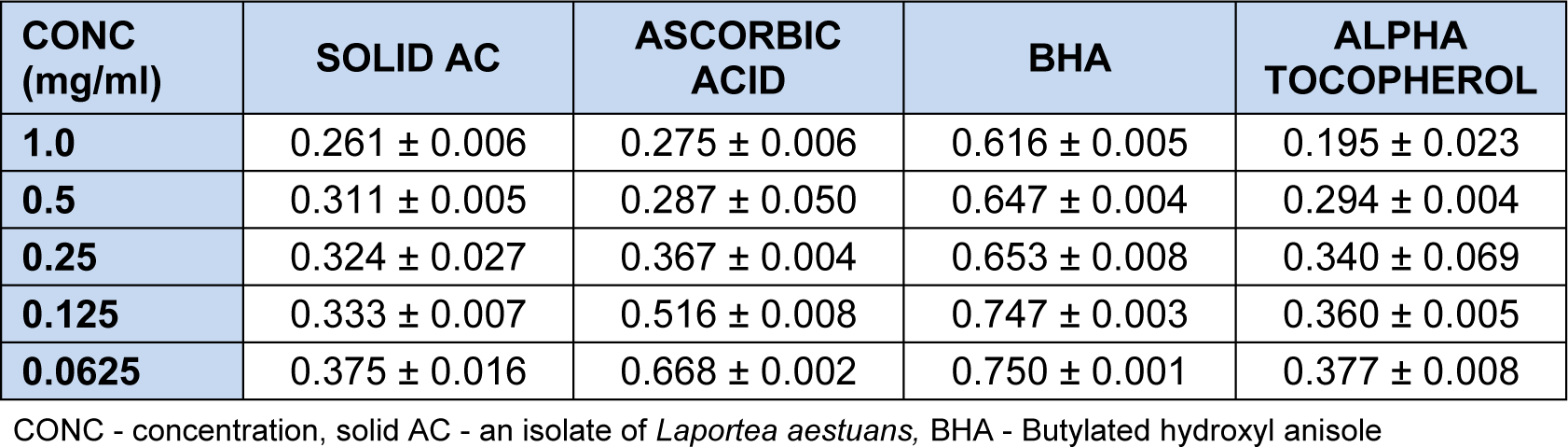
The amount of peroxide which oxidized ferrous chloride (FeCl2) to a reddish ferric chloride (FeCl3) pigment was determined. Here absorbance values increases as the concentration decreases. Also, the concentration of peroxide decreases as the antioxidant activity increases. At 1.0- 0.0625 mg/ml, Solid AC showed antioxidant activities in a concentration dependent manner (Table 4(Tab. 4)). And activity was significant and better (65.23 %) at 1.0 mg/ml than the activity of BHA (17.95 %).
Antimicrobial screening of solid AC
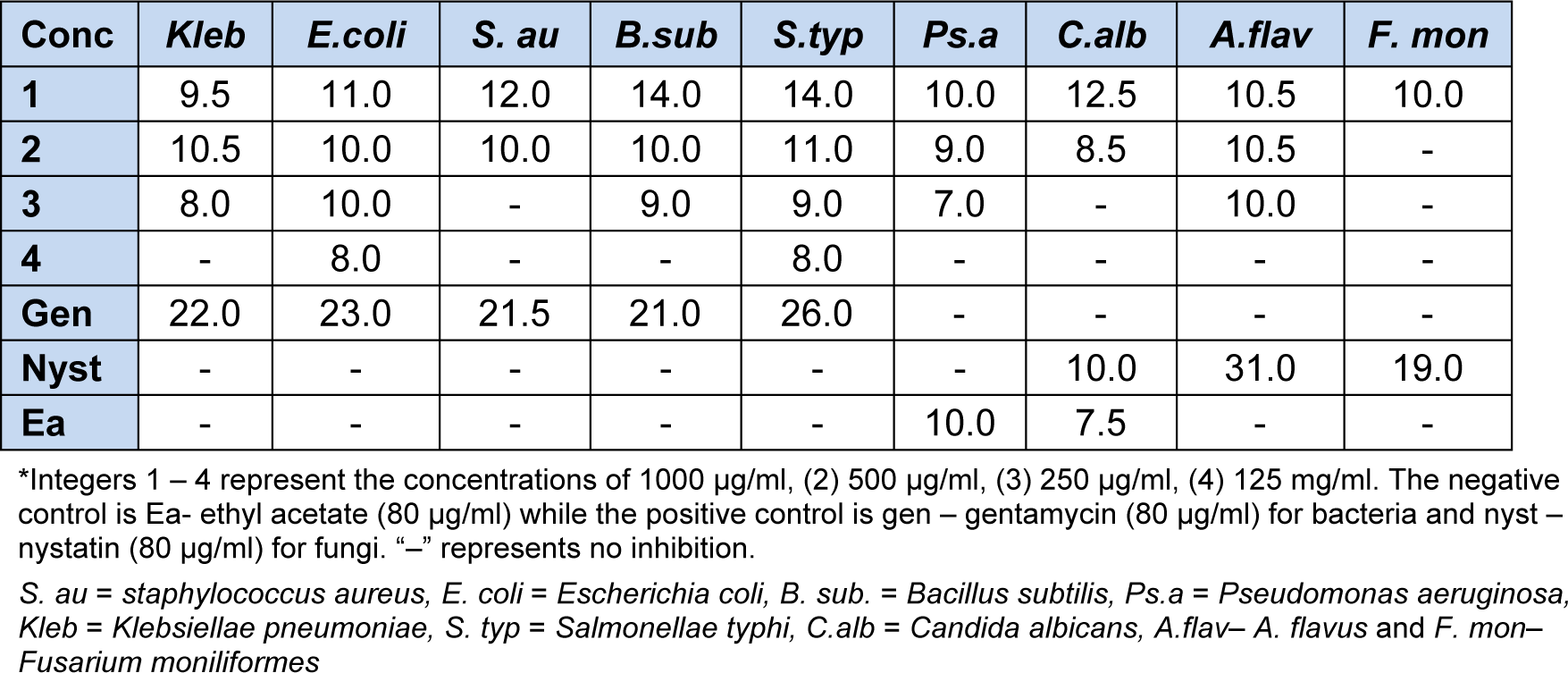
The activity of solid AC against bacteria and fungi strains was tested at various concentrations. The zones of inhibition were measured and reported in Table 5(Tab. 5).
Discussion
The UV absorption spectra measured between wavelengths 190 nm and 400 nm showed the presence of chromophore in solid AB at λmax 207.78 nm and 254.48 nm. The IR spectrum of solid AB exhibits absorption at 3432 cm-1, a broad band and intense peak which corresponds to an O-H group which is hydrogen bonding. The C-H stretch of methyl group absorbed at 2908.43 cm-1. The absorption at 1635.07 cm-1 corresponds to C = C stretch. The several band observed within the range of 1402 - 568 cm-1 were due to C-H aliphatic out of plane bending, C - O, C - C bonds stretching vibrations in the fingerprint region. The 1H NMR spectrum showed a methyl proton signal which resonated at δ 0.92 ppm and hydroxylic proton at δ 3.75 ppm corresponding to hydroxyl carbon at δ 76.374 ppm. The excessively tall singlet signal at δ 1.4 ppm implies that many CH3 of the same chemical environment are present which corresponds to peaks at δ 14.143 - 29.371 ppm in 13C NMR and 63.122 ppm corresponds to C-H of alkene. It was therefore concluded based on information obtained from 1H NMR and 13C NMR spectroscopy that the compound was (4E)-3,6-dimethylhep-4-en-3-ol.
The UV spectrum of AC showed absorption at λ 210.79 nm. The IR (KBr cm-1) of crystal AC showed a broad band and intense peak at 3432 cm-1 which corresponds to an O - H group which is hydrogen bonding. The C - H stretch of methyl group absorbed at 2939.13 cm-1. The absorption at 1657.03 cm-1 corresponds to C = C stretch. There is also absorption at 1250.42 cm-1 that indicated a C - O stretching vibration. The peak at 1371.28 cm-1 corresponds to C - H deformation of sp3. Characterization and composition of chrysen-2-ol is therefore supported by IR spectroscopy data of hydroxyl band at 3432.00 cm-1 typical of a chrysen-2-ol nucleus and the broad peaks representing the free 6-methyl observed on the derivative at 2939.13 cm-1. The NMR spectrum was in accordance with the proposed structure. The OH signal occurring at 5.4 is a singlet. The 13C spectrum revealed two signals characteristic of an olefinic carbon which is quaternary carbon at δ 140.39 and 138.334, a protonated carbon. Oxygenated carbon at δ 71.776 indicated the presence of one hydroxyl group and the signals between δ 23 and 24 ppm are the angular methyl carbon. The two methine signals at δ 129.241 and δ 121.715 ppm, correlating with proton signal at δ 5.2 indicate the presence of double bond at ring. The signals between δ 23.046 and 56.745 correspond with the several proton peaks between δ 0.9 and 1.9 which indicate the presence of carbon and proton of methine and methylene group of the cyclic ring. The compound is suspected to be
1,2,3,4,4a,4b,5,6,6a,7,8,9,10,10a,10b,11-hexadecahydro-1,1,6a,10b-tetramethyl-7-((E)-4,7-dimethyloct-5-enyl)chrysen-2-ol (AC). The UV, IR and NMR spectra of the compounds support similar compounds in literature. Based on the observed spectra characteristic of the two compounds, it was suspected that
4E) - 3, 6-dimethylhep-4-en-3-ol is a cleavage product of
1,2,3,4,4a,4b,5,6,6a,7,8,9,10,10a,10b,11-hexadecahydro-1,1,6a,10b-tetramethyl-7-((E)-4,7-dimethyloct-5-enyl)chrysen-2-ol
The result of the brine shrimp lethality test showed that solid AC with LC50 values 1581233000.0 µg/ml was not toxic as it had LC50 value greater than 1000 µg/ml. In vitro antioxidant screening by the three complementary tests, namely DPPH free radical scavenging effect, scavenging effect on hydrogen peroxide and scavenging effect on ferric thiocynate of AC showed an overall activity comparable to the antioxidant standards. It was observed that the % inhibition increased with decrease in concentration during the DPPH scavenging activity. At 1.0 mg/ml, the % inhibition was 37.46 % while that of the lowest concentration (0.0625 mg/ml) was 64.73 %. It was also observed that the AC had better inhibition when compared to α-tocopherol.
At 0.125 mg/ml and 0.0625 mg/ml, solid AC also showed better activity than rutin. The scavenging effect of solid AC on hydrogen peroxide showed that the % inhibition increased with decrease in concentration. At the highest concentration (1.0 mg/ml), AC had % inhibition of 64.47 % on hydrogen peroxide and 99.23 % at the least concentration (0.0625 mg/ml). Solid AC showed better inhibition than ascorbic acid at 0.25 mg/ml, 0.125 mg/ml and 0.0625 mg/ml and more inhibitive effect than butylated hydroxyl anisole (BHA) and α-tocopherol at 0.125 mg/ml and 0.0625 mg/ml. The result of the scavenging effect of solid AC on ferric thiocynate showed that the % inhibition increased with increase in concentration with the highest concentration 1.0 mg/ml having a % inhibition of 65.23 % and the least concentration which was 0.0625 mg/ml having a % inhibition of 50.00. It showed higher inhibition than butylated hydroxyl anisole (BHA), ascorbic acid and α-tocopherol at 0.25 -0.0625 mg/ml. Activity observed was linked to the presence of chrysen-2-ol nucleus.
Antimicrobial screening result showed that solid AC was active on all the tested organisms at 1000 μg/ml, but as the concentration decreases the diameter of inhibition decreases. AC had better inhibition on C. albicans (12.5 mm) than nystatin (10.0 mm) indicating a selective inhibition on fungal strains.
Conclusion
Laportea aestuans is an herb used in Africa to treat diseases related to oxidative stress and microbial infections locally. Two compounds
(4E)-3,6-dimethylhep-4-en-3-ol (AB) and 1,2,3,4,4a,4b,5,6,6a,7,8,9,10,10a,10b,11-hexadecahydro-1,1,6a,10b-tetramethyl-7-((E)-4,7-dimethyloct-5-enyl)chrysen-2-ol (AC) were isolated and characterized using data obtained from UV/Visible, IR, 1H and 13C NMR spectroscopies. AC was found to be non-toxic and possessed significant antioxidant activity when compared to standards; ascorbic acid, butylated hydroxyl anisole, rutin and α-tocopherol. The selective antimicrobial activity of AC confirmed its usefulness in the treatment of various infectious diseases caused by bacteria and fungi locally.
Acknowledgement
The authors gladly acknowledge the staff of Multi Research Central Science Laboratory, University of Ibadan, Oyo State and Central Science Laboratory Obafemi Awolowo University (OAU) Ile Ife, Osun State for the use of spectroscopic equipment. The authors will also like to thank Dr Idowu of Department of pharmaceutical Chemistry, OAU, Ile Ife and staff of the department of Microbiology University of Ibadan, Nigeria for assisting in carrying out the antimicrobial analysis.
References
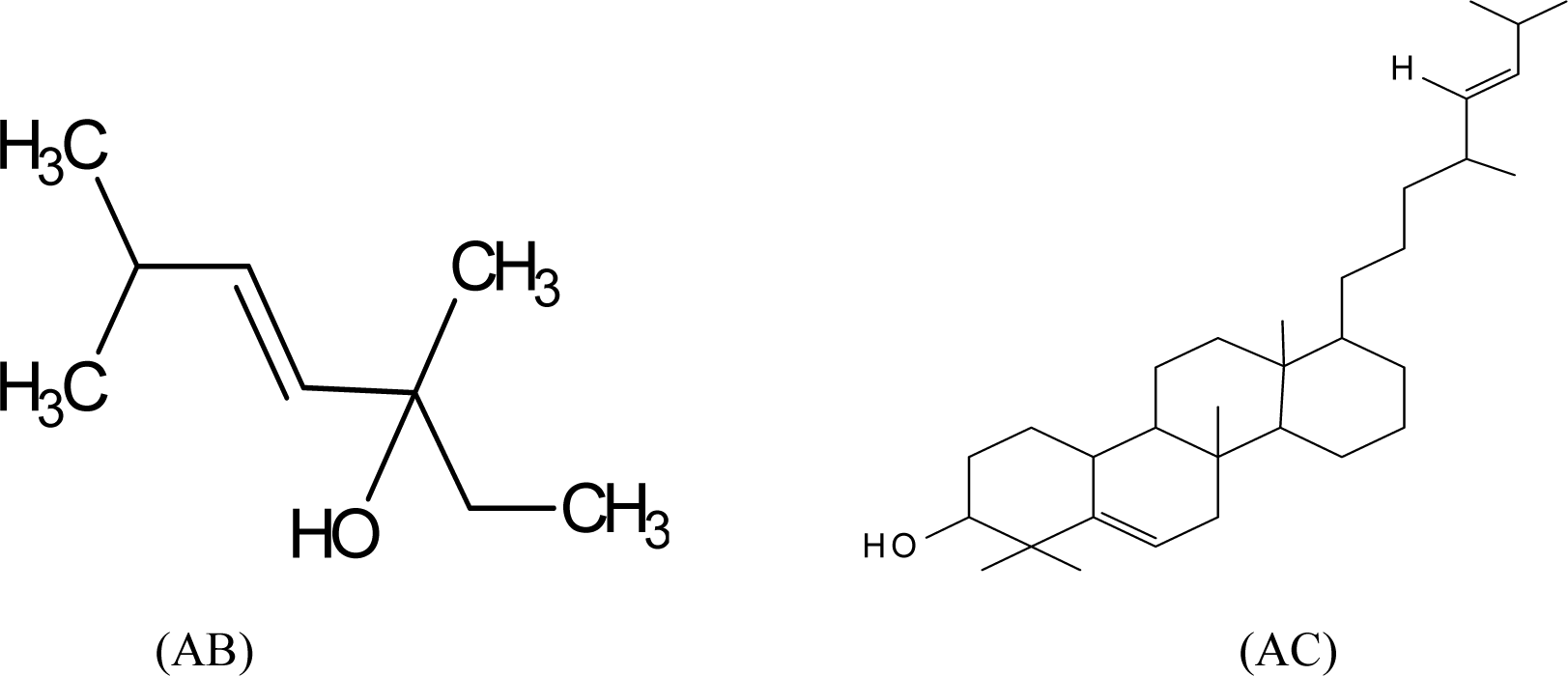
Figure 1: Structures of (4E)-3,6-dimethylhep-4-en-3-ol (AB) and 1,2,3,4,4a,4b,5,6,6a,7,8,9,10,10a, 10b,11-hexadecahydro-1,1,6a,10b-tetramethyl-7-((E)-4,7-dimethyloct-5-enyl)chrysen-2-ol (AC)
(4E)-3,6-dimethylhep-4-en-3-ol (AB): Molecular formulae C9H18O, white solid substance. Yield: (8.3 mg) 77.7 %. M.pt: 760C-780C. Rf: 0.69 (EtOAc-Hexane, 1:4). UV [EtOH] nm (log ε): 207.78 (3.2508) and 254.48 (0.78588). IR (KBr) Vmax cm-1: 3432 (O-H), 2908.43 (C - H), 1635.03 (C = C). 1401.95, 1257.74, 1055.39, 771.20, 567.75 (fingerprint region, C-H aliphatic out of plane bending, C - O, C - C etc); 1H NMR (200 MHz; CDCl3): 3.75 (1H, m, O-H and 2H, C=C-H overlap), 1.6 (2H, m, CH2), 1.4 (10H, m, CH3-), 0.9 (3H, t, CH3). 13C-NMR (50 MHz; CDCl3): 76.374 (C-3, C-O), 63.122 (C-H, alkene C-4), 32.812(C-5, C-H, alkene), 31.934(C-6, C-H), 29.708 (C-2, CH2), 29.371(C-9, CH3), 25.740 (C-7, CH3), 22.694 (C-8, CH3), 14.143 (C-1, CH3).
1,2,3,4,4a,4b,5,6,6a,7,8,9,10,10a,10b,11-hexadecahydro-1,1,6a,10b-tetramethyl-7-((E)-4,7-dimethyloct-5-enyl)chrysen-2-ol (Solid AC): Molecular formulae C31H52O, white crystalline solid. Yield: (0.2337 g) 91.2 %. M.pt: 1310C-1320C, Rf: 0.94 (EtOAc-Hexane, 1:4). UV [EtOH] nm (log ε): 210.79 (2.4780). IR (KBr) Vmax cm-1 : 3432.00 (O-H), 2939.13 (C-H), 1760.78-1641.17(C=C), 1250.42, 1058.45 and 573.00 (fingerprint region, C-H aliphatic , C - O, C - C); 1H NMR (200 MHz; CDCl3): 5.4 (1H, O-H, s), 5.2 (1H, m, ring C=H), 3.5 (1H, m, C=H alkene), 2.3 (1H, m C=H alkene) and 0.9-1.9 (48H, m, CH2 and CH3), 13C-NMR (50 MHz; CDCl3): 140.743 (C-5, unsaturated sp2 ring system), 121.707 (C-6, unsaturated sp2 ring system), 129.234 (C-28, unsaturated sp2, alkene), 138.327 (C-29, unsaturated sp2, alkene), 71.776, (C-3, C-O). 56.841(C-14), 56.738(C-9), 56.021(C-4), 55.918(C-8), 51.232(C-13), 50.105(C-18), 45.800(C-7), 42.301(C-26), 42.271(C-25), 42.198(C-12), 40.514(C-10), 39.753(C-30), 39.665(C-23), 37.234(C-2), 36.487(C-17), 36.136(C-1), 33.910(C-24), 31.875(C-15), 31.626(C-16), 29.108(C-31), 28.932(C-32), 28.244(C-11), 26.018(C-27), 25.418(C-19), 24.363(C-20), 24.290(C-21), 23.046(C-22).
[*] Corresponding Author:
Ganiyat K. Oloyede, Natural products/Medicinal Chemistry Unit, Department of Chemistry, University of Ibadan, Nigeria; Telephone: +234 803 562 2238, eMail: oloyedegk@gmail.com