Letter to the editor
Metabolic dyshomeostasis by organophosphate insecticides: insights from experimental and human studies
Apurva Kumar Ramesh Joshi1, Bindhu Omana Sukumaran1
1Department of Biochemistry, School of Sciences, Jain University, Bangalore, Karnataka, India 560041
EXCLI J 2019;18:Doc479
Dear Editor,
Organophosphate insecticides (OPI), derived from phosphoric, phosphonic or phosphinic acids, find application as agents for controlling insect pest populations. OPIs elicit their characteristic toxicity by phosphorylating and inhibiting the enzyme, acetylcholinesterase (AChE). Cholinergic stress resulting from overstimulation of nicotinic- and muscarinic acetylcholine receptors is the chief mechanism of acute toxicity of OPI (Fukuto, 1990[9]; Sogorb and Vilanova, 2002[31]; Abou-Donia, 2003[2]; Costa, 2006[7]). The ubiquitous nature of AChE and its conserved physiological role in the regulation of neurotransmission means that non-target animals (including humans) are at risk of adverse outcomes in the event of exposure to OPI. Neurotoxicity, characterized by cholinergic and non-cholinergic outcomes, is the most studied aspect of OPI toxicity. However, it is now unequivocally recognized that the toxicity of OPIs goes beyond the realm of neurotoxicity.
A large number of animal studies explicitly demonstrate that OPIs have the propensity to cause hyperglycemia, perturbations in carbohydrate metabolism and endocrine dysregulations. Repeated exposure to OPI precipitates insulin resistance (studies listed in Table 1(Tab. 1); References in Table 1: Abdollahi et al., 2004[1]; Acker and Nogueira, 2012[3]; Fuentes-Delgado et al., 2018[8]; Hamza et al., 2014[11]; Joshi and Rajini, 2009[14]; Joshi and Rajini, 2012[15]; Joshi et al., 2012[13]; Kamath et al., 2008[17]; Lasram et al., 2008[18]; Lasram et al., 2015[19]; Liang et al., 2019[20]; Mostafalou et al., 2012[24]; Nagaraju and Rajini, 2016[26]; Nagaraju et al., 2015[25]; Ribeiro et al., 2016[29]; Salek-Maghsoudi et al., 2019[30]; Velmurugan et al., 2013[34]; Velmurugan et al., 2017[33]; Yousefizadeh et al., 2019[36]), a key component of metabolic syndrome. Extrapolating the outcomes of animal experimentation to the human situation is a challenging task. Experimental studies often employ doses much higher than doses of environmental relevance. However, several studies clearly (listed in Table 2(Tab. 2); References in Table 2: Amanvermez et al., 2010[4]; Ather et al., 2008[5]; Çolak et al., 2014[6]; Gifford et al., 2019[10]; Hui, 1983[12]; Montgomery et al., 2008[21]; Moon et al., 2016[22]; Moore and James, 1981[23]; Raafat et al., 2012[27]; Rao and Raju, 2016[28]; Sudhir et al., 2013[32]; Velmurugan et al., 2017[33]; Weizman and Sofer, 1992[35]; Yurumez et al., 2007[37]) establish that OPI exposure elicits metabolic dyshomeostasis in human subjects. A recent study demonstrates that the incidence of diabetes among Thai farmers positively correlates with OPIs such as chlorpyrifos, dicrotophos, dichlorvos, ethyl-p-nitrophenyl, mevinphos, monocrotophos and methamidophos (Juntarawijit and Juntarawijit, 2018[16]). Thus, it is evident that OPI exposure is a clear risk factor for metabolic dysregulations among those who are occupationally exposed. One has to take cognizance of the fact that levels of exposure to OPI among occupationally exposed people are likely to be higher than the general population. However, a recent study reveals that glycated hemoglobin levels correlate with plasma levels of OPI (due to environmental exposure), but not with AChE activity (Velmurugan et al., 2017[33]). This indicates that low-level OPI exposure may cause metabolic dysregulations. Hence, we believe that further studies are needed to evaluate the effects of low-level, chronic non-occupational exposure to OPI on metabolic health.
Acknowledgements
Authors are thankful to Jain University, Bangalore for the support.
Conflict of interest
The authors declare no conflict of interest.
References
1.
Abdollahi M, Donyavi M, Pournourmohammadi S, Saadat M. Hyperglycemia associated with increased hepatic glycogen phosphorylase and phosphoenolpyruvate carboxykinase in rats following subchronic exposure to malathion. Comp Biochem Physiol Part C Toxicol Pharmacol. 2004;137:343–7.2.
Abou-Donia MB. Organophosphorus ester-induced chronic neurotoxicity. Arch Environ Health. 2003;58:484–97.3.
Acker CI, Nogueira CW. Chlorpyrifos acute exposure induces hyperglycemia and hyperlipidemia in rats. Chemosphere. 2012;89:602–8. 4.
Amanvermez R, Baydın A, Yardan T, Başol N, Günay M. Emergency laboratory abnormalities in suicidal patients with acute organophosphate poisoning. Turkish J Biochem. 2010;35:29–34.5.
Ather NA, Ara J, Khan EA, Sattar RA, Durrani R. Acute organophosphate insecticide poisoning. J Surg Pakistan. 2008;13:71–4. 6.
Çolak Ş, Erdoğan MÖ, Baydin A, Afacan MA, Kati C, Duran L. Epidemiology of organophosphate intoxication and predictors of intermediate syndrome. Turkish J Med Sci. 2014;44:279–82. 7.
Costa LG. Current issues in organophosphate toxicology. Clin Chim Acta. 2006;366:1–13. 8.
Fuentes-Delgado VH, Martínez-Saldaña MC, Rodríguez-Vázquez ML, Reyes-Romero MA, Reyes-Sánchez JL, Jaramillo-Juárez F. Renal damage induced by the pesticide methyl parathion in male Wistar rats. J Toxicol Environ Health Part A. 2018;81:130–41. 9.
Fukuto TR. Mechanism of action of organophosphorus and carbamate insecticides. Environ Health Perspect. 1990;87:245–54. 10.
Gifford RM, Chathuranga U, Lamb T, Verma V, Sattar MA, Thompson A, et al. Short-term glucose dysregulation following acute poisoning with organophosphorus insecticides but not herbicides, carbamate or pyrethroid insecticides in South Asia. Clin Toxicol. 2019;57:254–64.11.
Hamza RZMM, Diab AE, El-Aziz E-SAA. Hyperglycemic effect of chlorpyrifos , profenofos and possible ameliorative role of propolis and ginseng. Sci Agric PSCI Publ. 2014;9–14.12.
Hui KS. Metabolic disturbances in organophosphate insecticide poisoning. Arch Pathol Lab Med. 1983;107:154. 13.
Joshi AKR, Nagaraju R, Rajini PS. Insights into the mechanisms mediating hyperglycemic and stressogenic outcomes in rats treated with monocrotophos, an organophosphorus insecticide. Toxicology. 2012;294:9–16. 14.
Joshi AKR, Rajini PS. Reversible hyperglycemia in rats following acute exposure to acephate, an organophosphorus insecticide: role of gluconeogenesis. Toxicology. 2009;257:40–5. 15.
Joshi AKR, Rajini PSR. Hyperglycemic and stressogenic effects of monocrotophos in rats: evidence for the involvement of acetylcholinesterase inhibition. Exp Toxicol Pathol. 2012;64:115–20. 16.
Juntarawijit C, Juntarawijit Y. Association between diabetes and pesticides: a case-control study among Thai farmers. Environ Health Prev Med. 2018;23:3. 17.
Kamath V, Joshi AKR, Rajini PS. Dimethoate induced biochemical perturbations in rat pancreas and its attenuation by cashew nut skin extract. Pestic Biochem Physiol. 2008;90:58–65. 18.
Lasram MM, Annabi AB, Rezg R, Elj N, Slimen S, Kamoun A, et al. Effect of short-time malathion administration on glucose homeostasis in Wistar rat. Pestic Biochem Physiol. 2008;92:114–9. 19.
Lasram MM, Bouzid K, Douib IB, Annabi A, El Elj N, El Fazaa S, et al. Lipid metabolism disturbances contribute to insulin resistance and decrease insulin sensitivity by malathion exposure in Wistar rat. Drug Chem Toxicol. 2015;38:227–34. 20.
Liang Y, Zhan J, Liu D, Luo M, Han J, Liu X, et al. Organophosphorus pesticide chlorpyrifos intake promotes obesity and insulin resistance through impacting gut and gut microbiota. Microbiome. 2019;7:19. 21.
Montgomery MP, Kamel F, Saldana TM, Alavanja MCR, Sandler DP. Incident diabetes and pesticide exposure among licensed pesticide applicators: Agricultural Health Study, 1993-2003. Am J Epidemiol. 2008;167:1235–46. 22.
Moon JM, Chun BJ, Cho YS. Hyperglycemia at presentation is associated with in hospital mortality in non-diabetic patient with organophosphate poisoning. Clin Toxicol. 2016;54:252–8.23.
Moore PG, James OF. Acute pancreatitis induced by acute organophosphate poisoning. Postgrad Med J. 1981;57:660–2. 24.
Mostafalou S, Eghbal MA, Nili-Ahmadabadi A, Baeeri M, Abdollahi M. Biochemical evidence on the potential role of organophosphates in hepatic glucose metabolism toward insulin resistance through inflammatory signaling and free radical pathways. Toxicol Ind Health. 2012;28:840–51. 25.
Nagaraju R, Joshi AKR, Rajini PS. Organophosphorus insecticide, monocrotophos, possesses the propensity to induce insulin resistance in rats on chronic exposure. J Diabetes. 2015;7:47–59. 26.
Nagaraju R, Rajini P. Adaptive response of rat pancreatic β-cells to insulin resistance induced by monocrotophos: Biochemical evidence. Pestic Biochem Physiol. 2016;134:39–48. 27.
Raafat N, Abass MA, Salem HM. Malathion exposure and insulin resistance among a group of farmers in Al-Sharkia governorate. Clin Biochem. 2012;45:1591–5. 28.
Rao R, Raju G. Random blood sugar levels and pseudocholinesterase levels their relevance in organophosphorus compound poisoning. Int J Community Med Public Health. 2016;3:2757–61. 29.
Ribeiro TA, Prates KV, Pavanello A, Malta A, Tófolo LP, Martins IP, et al. Acephate exposure during a perinatal life program to type 2 diabetes. Toxicology. 2016;372:12–21. 30.
Salek-Maghsoudi A, Hassani S, Momtaz S, Shadboorestan A, Ganjali MR, Ghahremani MH, et al. Biochemical and molecular evidence on the role of vaspin in early detection of the insulin resistance in a rat model of high-fat diet and use of diazinon. Toxicology. 2019;411:1–14.31.
Sogorb MA, Vilanova E. Enzymes involved in the detoxification of organophosphorus, carbamate and pyrethroid insecticides through hydrolysis. Toxicol Lett. 2002;128:215–28. 32.
Sudhir U, Chandrashekar, Pai R, Sunil H, Rao M, Kempegowda P. Glycemic changes in acute anticholinesterase insecticide poisoning. West Lond Med J. 2013;5:27–33. 33.
Velmurugan G, Ramprasath T, Swaminathan K, Mithieux G, Rajendhran J, Dhivakar M, et al. Gut microbial degradation of organophosphate insecticides-induces glucose intolerance via gluconeogenesis. Genome Biol. 2017;18:8. 34.
Velmurugan G, Venkatesh Babu DD, Ramasamy S. Prolonged monocrotophos intake induces cardiac oxidative stress and myocardial damage in rats. Toxicology. 2013;307:103–8. 35.
Weizman Z, Sofer S. Acute pancreatitis in children with anticholinesterase insecticide intoxication. Pediatrics. 1992;90:204–6. 36.
Yousefizadeh S, Farkhondeh T, Samarghandian S. Age-related diazinon toxicity impact on blood glucose, lipid profile and selected biochemical indices in male rats. Curr Aging Sci. 2019, epub ahead of print.37.
Yurumez Y, Durukan P, Yavuz Y, Ikizceli I, Avsarogullari L, Ozkan S, et al. Acute organophosphate poisoning in university hospital emergency room patients. Intern Med. 2007;46:965-9.
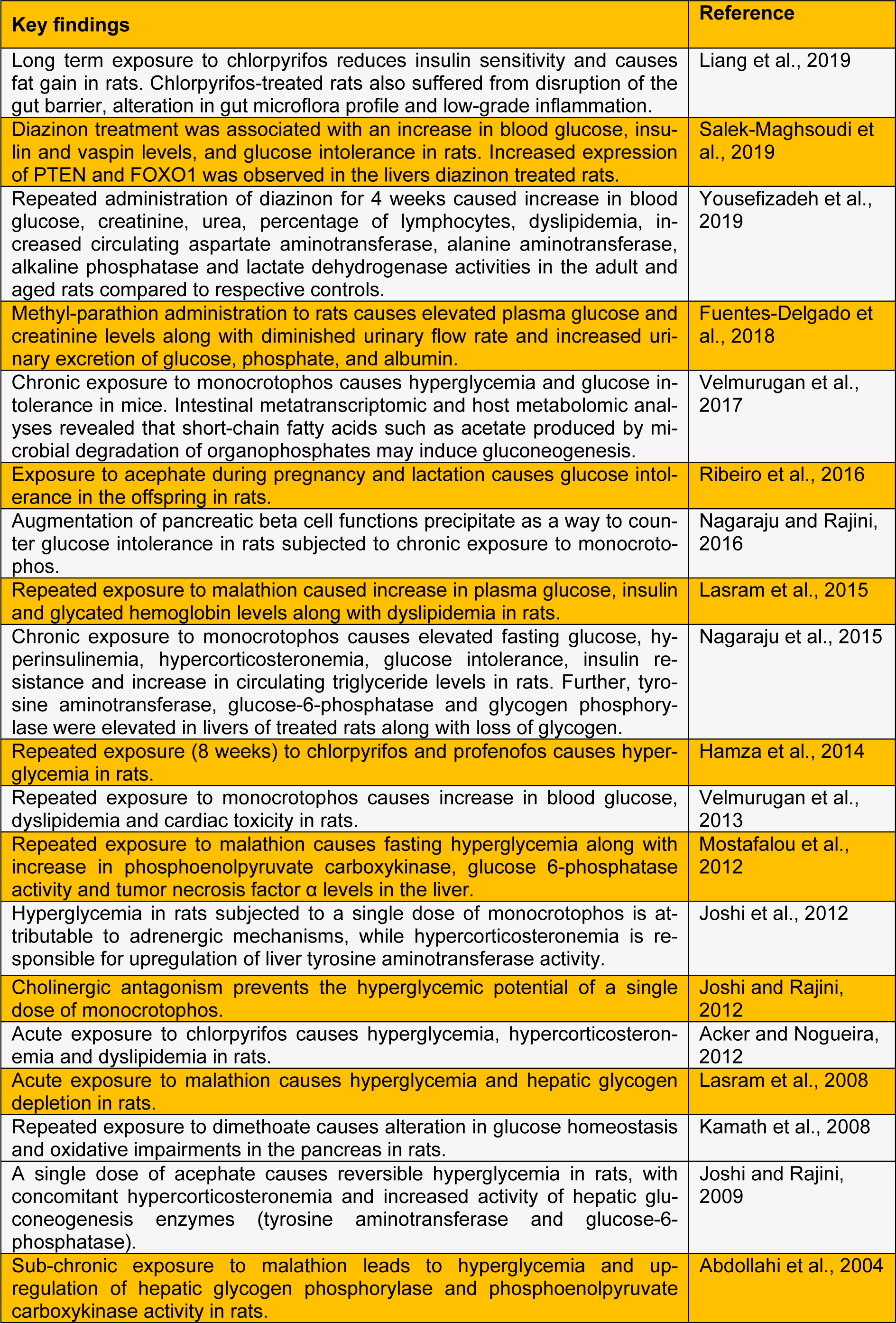
Table 1: Experimental studies reporting metabolic dysregulations caused by organophosphate insecticides in rodent models
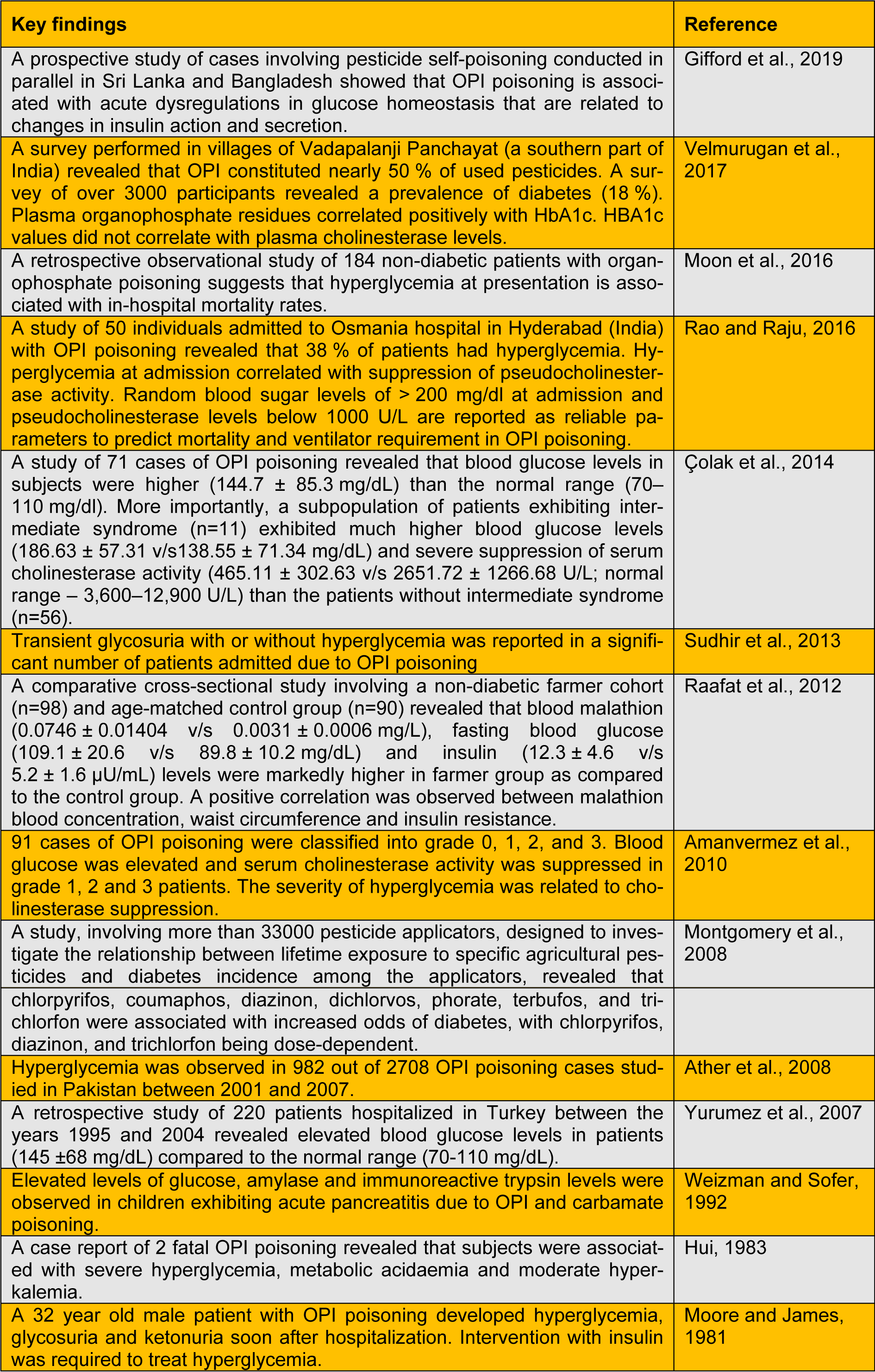
Table 2: Studies that demonstrate the link between exposure to organophosphate insecticides and metabolic dysregulations in human subjects

