Letter to the editor
A recent overview on sulforaphane as a dietary epigenetic modulator
Tae Kyung Hyun1
1Department of Industrial Plant Science and Technology, College of Agricultural, Life and Environmental Sciences, Chungbuk National University
EXCLI J 2020;19:Doc131
Dear Editor,
Gene expression is mediated by chromatin epigenetic changes, including DNA methylation, histone modifications, promoter-enhancer interactions, and non-coding RNA (microRNA and long non-coding RNA)-mediated regulation (Chen et al., 2017[5]). Approximately 50 % of all tumor suppressor genes are inactivated through epigenetic modifications, rather than by genetic mechanisms, in sporadic cancers (Meeran et al., 2010[12]; Su et al., 2018[16]). Accumulating evidence suggests that epigenetic modulators are important tools to improve the efficacy of disease prevention strategies (Ratovitski, 2017[14]; Carlos-Reyes et al., 2019[4]; Hassan et al., 2019[8]).
Sulforaphane ([1-isothioyanato-4-(methyl-sulfinyl)butane], SFN) is a naturally occurring, sulfur-containing isothiocyanate derivative that is found in the seeds and sprouts of cruciferous vegetables such as broccoli, cabbage, cauliflower, and kale (Vanduchova et al., 2019[19]). Because SFN induces the nuclear factor erythroid 2-related factor 2 (Nrf2)-antioxidant response element pathway that induces the cellular defense against oxidative stress (Trio et al., 2016[18]), SFN has received increased attention because it acts as an antioxidant, antimicrobial, anti-inflammatory, and anticancer agent (Vanduchova et al., 2019[19]). Various mechanisms, including apoptosis activation, nuclear factor-κB pathway inhibition, and cell cycle arrest induction, have been proposed to explain the beneficial effects of SFN in preventing multiple types of cancer (Tortorella et al., 2015[17]). Indeed, the increasing attention of SFN as an epigenetic modulator continues to contribute to new developments in clinical trials.
This letter presents a summary of key recent studies investigating the function of SFN as an epigenetic modulator in several human diseases (Table 1(Tab. 1); References in Table 1: Abbas et al., 2016[1]; Ali Khan et al., 2015[2]; Cao et al., 2018[3]; Fisher et al., 2016[6]; Gao et al., 2018[7]; Lewinska et al., 2017[9]; Li et al., 2019[10]; Lubecka-Pietruszewska et al., 2015[11]; Okonkwo et al., 2018[13]; Royston et al., 2018[15]; Yang et al., 2015[20]; Yuan et al., 2018[21]; Zhao et al., 2018[22]; Zhou et al., 2019[23]). I believe that this letter will stimulate future research on the development of SFN as an epigenetic modulator for successful chemo-prevention and alternative therapeutic approaches.
Conflict of interest
The author declares no conflict of interest.
References
1.
Abbas A, Hall JA, Patterson WL 3rd, Ho E, Hsu A, Al-Mulla F, Georgel PT. Sulforaphane modulates telomerase activity via epigenetic regulation in prostate cancer cell lines. Biochem Cell Biol. 2016;94:71-81.2.
Ali Khan M, Kedhari Sundaram M, Hamza A, Quraishi U, Gunasekera D, Ramesh L, Goala P, Al Alami U, Ansari MZ, Rizvi TA, Sharma C, Hussain A. Sulforaphane reverses the expression of various tumor suppressor genes by targeting DNMT3B and HDAC1 in human cervical cancer cells. Evid Based Complement Alternat Med. 2015;2015:412149.3.
Cao C, Wu H, Vasilatos SN, Chandran U, Qin Y, Wan Y, Oesterreich S, Davidson NE, Huang Y. HDAC5-LSD1 axis regulates antineoplastic effect of natural HDAC inhibitor sulforaphane in human breast cancer cells. Int J Cancer. 2018;143:1388-401.4.
Carlos-Reyes Á, López-González JS, Meneses-Flores M, Gallardo-Rincón D, Ruíz-García E, Marchat LA, Astudillo-de la Vega H, Hernández de la Cruz ON, López-Camarillo C. Dietary compounds as epigenetic modulating agents in cancer. Front Genet. 2019;10:79.5.
Chen Z, Li S, Subramaniam S, Shyy JY, Chien S. Epigenetic regulation: a new frontier for biomedical engineers. Annu Rev Biomed Eng. 2017;19:195-219.6.
Fisher ML, Adhikary G, Grun D, Kaetzel DM, Eckert RL. The Ezh2 polycomb group protein drives an aggressive phenotype in melanoma cancer stem cells and is a target of diet derived sulforaphane. Mol Carcinog. 2016;55:2024-36.7.
Gao L, Cheng D, Yang J, Wu R, Li W, Kong AN. Sulforaphane epigenetically demethylates the CpG sites of the miR-9-3 promoter and reactivates miR-9-3 expression in human lung cancer A549 cells. J Nutr Biochem. 2018;56:109-15.8.
Hassan FU, Rehman MS, Khan MS, Ali MA, Javed A, Nawaz A, Yang C. Curcumin as an alternative epigenetic modulator: mechanism of action and potential effects. Front Genet. 2019;10:514.9.
Lewinska A, Adamczyk-Grochala J, Deregowska A, Wnuk M. Sulforaphane-induced cell cycle arrest and senescence are accompanied by DNA hypomethylation and changes in microrna profile in breast cancer cells. Theranostics. 2017;7:3461-77.10.
Li Y, Yuan F, Wu T, Lu L, Liu J, Feng W, Chen SY. Sulforaphane protects against ethanol-induced apoptosis in neural crest cells through restoring epithelial-mesenchymal transition by epigenetically modulating the expression of Snail1. Biochim Biophys Acta Mol Basis Dis. 2019;1865:2586-94. 11.
Lubecka-Pietruszewska K, Kaufman-Szymczyk A, Stefanska B, Cebula-Obrzut B, Smolewski P, Fabianowska-Majewska K. Sulforaphane alone and in combination with clofarabine epigenetically regulates the expression of DNA methylation-silenced tumour suppressor genes in human breast cancer cells. J Nutrigenet Nutrigenomics. 2015;8:91-101.12.
Meeran SM, Ahmed A, Tollefsbol TO. Epigenetic targets of bioactive dietary components for cancer prevention and therapy. Clin Epigenetics. 2010;1:101-16.13.
Okonkwo A, Mitra J, Johnson GS, Li L, Dashwood WM, Hegde ML, Yue C, Dashwood RH, Rajendran P. Heterocyclic analogs of sulforaphane trigger DNA damage and impede DNA repair in colon cancer cells: interplay of HATs and HDACs. Mol Nutr Food Res. 2018;62:e1800228. 14.
Ratovitski EA. Anticancer natural compounds as epigenetic modulators of gene expression. Curr Genomics. 2017;18:175-205.15.
Royston KJ, Paul B, Nozell S, Rajbhandari R, Tollefsbol TO. Withaferin A and sulforaphane regulate breast cancer cell cycle progression through epigenetic mechanisms. Exp Cell Res. 2018;368:67-74.16.
Su X, Jiang X, Meng L, Dong X, Shen Y, Xin Y. Anticancer activity of sulforaphane: the epigenetic mechanisms and the Nrf2 signaling pathway. Oxid Med Cell Longev. 2018;2018:5438179.17.
Tortorella SM, Royce SG, Licciardi PV, Karagiannis TC. Dietary sulforaphane in cancer chemoprevention: the role of epigenetic regulation and HDAC inhibition. Antioxid Redox Signal. 2015;22:1382-424.18.
Trio PZ, Fujisaki S, Tanigawa S, Hisanaga A, Sakao K, Hou DX. DNA microarray highlights Nrf2-mediated neuron protection targeted by wasabi-derived isothiocyanates in IMR-32 cells. Gene Regul Syst Bio. 2016;10:73-83.19.
Vanduchova A, Anzenbacher P, Anzenbacherova E. Isothiocyanate from broccoli, sulforaphane, and its properties. J Med Food. 2019;22:121-6.20.
Yang SF, Lee WJ, Tan P, Tang CH, Hsiao M, Hsieh FK, Chien MH. Upregulation of miR-328 and inhibition of CREB-DNA-binding activity are critical for resveratrol-mediated suppression of matrix metalloproteinase-2 and subsequent metastatic ability in human osteosarcomas. Oncotarget. 2015;6:2736-53.21.
Yuan F, Chen X, Liu J, Feng W, Cai L, Wu X, Chen SY. Sulforaphane restores acetyl-histone H3 binding to Bcl-2 promoter and prevents apoptosis in ethanol-exposed neural crest cells and mouse embryos. Exp Neurol. 2018;300:60-6.22.
Zhao F, Zhang J, Chang N. Epigenetic modification of Nrf2 by sulforaphane increases the antioxidative and anti-inflammatory capacity in a cellular model of Alzheimer's disease. Eur J Pharmacol. 2018;824:1-10.23.
Zhou JW, Wang M, Sun NX, Qing Y, Yin TF, Li C, Wu D. Sulforaphane-induced epigenetic regulation of Nrf2 expression by DNA methyltransferase in human Caco-2 cells. Oncol Lett. 2019;18:2639-47.
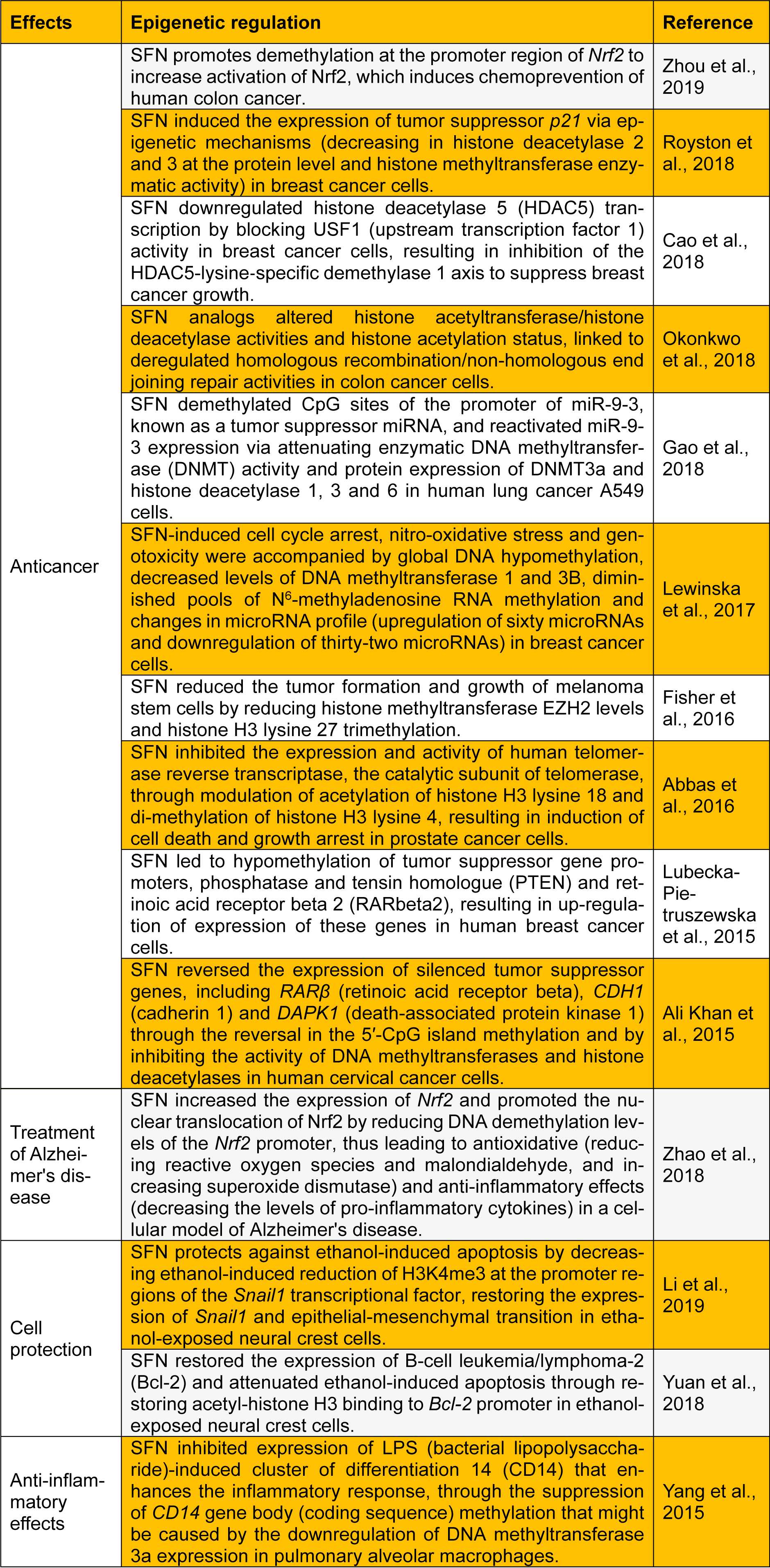
Table 1: Recent updates on sulforaphane (SFN) as a dietary epigenetic modulator
