Research article
Differential expression of Scinderin and Gelsolin in gastric cancer and comparison with clinical and morphological characteristics
Toktam Sadat Tavabe Ghavami1, Shiva Irani2, Reza Mirfakhrai3, Reza Shirkoohi4[*]
1Department of Genetics, Islamic Azad University, Tehran, Iran2Department of Biology, Islamic Azad University, Tehran, Iran
3Department of Medical Genetics, Shahid Beheshti University of Medical Sciences, Tehran, Iran
4Cancer Research Institute, Tehran University of Medical Sciences, Tehran, Iran
EXCLI J 2020;19:Doc750
Abstract
Gastric cancer is the first cause of cancer-related death in males and the second in female patients in Iran. Advanced cancer is usually associated with distant metastasis, which is uncontrollable. This study was conducted to compare the expression of Scinderin and Gelsolin genes between gastric cancer and adjacent normal tissue samples in Iranian patients in order to better understand the role of these genes in this disease and to assess them as potential gastric cancer diagnostic or prognostic biomarkers. This case-control study was conducted in 41 Iranian patients suffering from stage I to IV of Gastric Cancer diagnosed by pathologic and endoscopic tests. In this study, significant down-regulation of Gelsolin (p=0.001) and over-expression of Scinderin (p=0.001) were observed in tumor tissues compared to the adjacent normal tissues. The results of the present study showed decreased Gelsolin expression in patients above 40 years, while the relationship between Gelsolin expression and age was not significant; also, a significant increase was observed in Scinderin expression in patients above 40 years. Furthermore, Lymph node metastasis was observed in 59.52 % of the cases. The results showed that reduced Gelsolin and increased Scinderin expression were related to lymph node metastasis. Based on results, a significant association was observed between tumor size and Scinderin expression level. Furthermore, Gelsolin and Scinderin expressions were assessed in different grades and stages to determine the association of this gene with cancer progression. The result indicates significant alteration in Scinderin expression level of I and IV, II and IV, and III and IV stages. Although no significant association was observed between Scinderin expression level and GC grade, the mean Gelsolin expression showed a significant difference between grade II and III as well as grade I and IV. Based on our results, these genes would be potential biomarkers.
Keywords: gastric cancer, Gelsolin, Scinderin, expression, up-regulation, down-regulation
Introduction
Gastric cancer (GC) is one of the most prevalent cancers and a leading cause of oncologic death globally. In spite of recent advances in diagnosis and therapeutics, its mortality and incidence rates are still high due to a delayed diagnosis. Approximately 700,000 GC-related deaths are reported annually (Bertuccio et al., 2009[4]; Siegel et al., 2020[40]). It can be cured by surgery at early stages but most GC patients are detected in advanced and metastasis stages. Consequently, poor metastasis prognosis might hinder this mortality rate (Necula et al., 2019[33]; Sun et al., 2012[42]; Torre et al., 2015[46]).
Early metastasis detection might play a valuable role in decreasing the incidence and mortality rates of the disease. During metastasis, mesenchymal cells in the distant organs, become epithelial cells (mesenchymal-TO-epithelial cells transition: MET) and induces an irregular growth of cancer cells. The epithelial-to-mesenchymal transition (EMT), a pathologic phenomenon in cancer, plays an important role in tumor metastasis and progression (Lee et al., 2006[23]; Shirkoohi, 2013[38]).
EMT occurs during normal embryonic development, organ fibrosis, and tissue regeneration. In the first stage, its promotion will make metastasis a life-threatening stage of cancer while in the second stage, EMT would lead to organogenesis, which is necessary for all living organisms. Another phenomenon occurs during the wound healing process, which can result in fibrosis if it is not properly regulated. The stimuli were the same in two cases, but they were different in one. Understanding the cellular behavior in this process and what makes them invasive resistant stemness cells is very important for highlighting cancer treatment modalities (Ghazimoradi and Farivar, 2020[12]; Shirkoohi, 2013[38]).
Although many studies have been conducted to uncover the mysteries of cancer initiation, progression, and invasion at the molecular level, the exact molecular mechanism has not been fully elucidated yet. So, improving our knowledge of the molecular mechanisms underlying cancers might lead to discovering new diagnostic and therapeutic biomarkers (Lazăr et al., 2016[22]).
Since actin-binding proteins play an essential role in regulating actin polymerization, these proteins have been important in cancer research (Gross, 2013[14]). It has been observed that Gelsolin protein (a protein expressed in all mammalian tissues) plays an important role in actin reorganization and remodeling of apoptotic cells (Migocka-Patrza et al., 2019[32]; Shirkoohi et al., 2012[39]). In addition, Scinderin protein is one of the actin-binding and separating proteins that regulates the activity of cytoskeletal actin filaments (Trifaro et al., 2000[48]).
Gelsolin, which is encoded by the GSN gene, plays a crucial role in normal cell motility and morphology maintenance by severing, capping and nucleating actin filaments (Ditsch and Wegner, 1994[8]; Gremm and Wegner, 2000[13]; Ong et al., 2020[34]; Selden et al., 1998[37]). Gelsolin regulates Rac (a member of Rho family) that regulates actin filament organization. It has been shown that silencing Gelsolin correlates with Rac up-regulation and an increase in the amounts of F-actin in stress fibers, which may result in lamellipodia (Li et al., 2012[25]; Tanaka et al., 2006[43]; Trejo-Cerro et al., 2019[47]). Aberrant expression of Gelsolin has been observed in both human and animal cancer initiation (Asch et al., 1996[3]). Cancer cells may evade the apoptosis signaling pathway through Gelsolin down-regulation (Kwiatkowski, 1999[20]). Although a large body of evidence suggests that Gelsolin might act as a tumor suppressor gene, its up-regulation enhances metastasis in later cancer stages. Thus, Gelsolin plays critical roles in human malignancies from initiation to invasion and metastasis (Huang et al., 2015[17]). Stock et al. (2015[41]) examined the impact of Gelsolin on breast cancer cell migration. Moreover, they studied the relationship between this gene expression and metastasis-free survival in node-negative breast cancer. It is notable that over-expression of Gelsolin gene in MCF7 and MDA-DB-468 cell lines decrease cancer cells migration and metastasis while its knockdown had reverse impact. Tanaka et al. (2006[43]) reported that the expression of Gelsolin was indistinguishable in human mammary epithelial cells. Mielnicki et al. (1999[31]) demonstrated the negative regulation of Gelsolin expression in breast cancer during epigenetic mechanisms. It has been stated that over-expression of Gelsolin, as a tumor suppressor, prevents the growth of bladder, breast, and lung cancer cells (Sagawa et al., 2003[36]). It is notable that the increased amount of histone deacetylases depends on Gelsolin expression suppression in gastric cancer (Kim et al., 2004[19]). The relationship between decreased Gelsolin expression and tumor metastasis supports this idea (Li et al., 2009[26]). Shirkoohi et al. (2012[39]) introduced Gelsolin as a cytoskeletal tumor suppressor and reported that Gelsolin level expression affected cell differentiation. The controversy of Gelsolin role might be answered by its relationship with estrogen receptor. Stock et al. (2015[41]) state that patients with breast cancer whom their cancer are ER positive show a correlation among higher Gelsolin expression and lower tumor stage, grade, cell proliferation and higher metastasis-free survival rate, however ER negative patients have a converse relationship.
Furthermore, Scinderin is a member of the Gelsolin supper family that plays a key role in actin filament reconstruction in a Ca2+ dependent manner by its three actin-, two PIP2-, and two Ca2+-binding sites (Chen et al., 2014[5]; Dumitrescu Pene et al., 2005[10]; Lejen et al., 2002[24]). By controlling F-actin dynamics and cytoskeleton underneath the plasma membrane, Scinderin plays a significant role in vesicle translocation and endocytosis (Chen et al., 2014[5]; Dumitrescu Pene et al., 2005[10]). According to observations in different studies, Scinderin over-expression has been reported in many malignancies (Liu et al., 2015[28]; Qiao et al., 2018[35]). It has been suggested that Scinderin silencing could decrease cells proliferation, induce cancer cell cycle arrest and apoptosis. It is notable that knockdown of Scinderin led to the suppression of EGFR expression level. For these reasons, many researchers have devoted their studies on Scinderin silencing, as a therapeutic strategy, because it plays an important role in cancer cell growth and development (Chen et al., 2014[5]; Lai et al., 2018[21]; Lin et al., 2019[27]). Chen et al. (2014[5]) studied the effect of Scinderin silencing in the SGC-7901 cell line and found a significant decrease in cell migration. It has been reported that Scinderin silencing inhibits cell migration, filopodia formation, and cell invasion ability (Dumitrescu Pene et al., 2005[10]; Liu et al., 2016[30]).
The purpose of this study was to investigate the expression levels of Gelsolin and Scinderin in GC and normal tissue samples. In addition, the relationship between the expression levels of these genes and clinical features was also evaluated in these patients.
Materials and Methods
Sample collection
This case-control study was conducted in 41 Iranian patients suffering from stage I to IV of Gastric Cancer diagnosed by pathologic and endoscopic tests in Imam Khomeini Hospital (Tehran, Iran). The Ethics Committee of TUMS approved the project, and informed consent was obtained from all participants (34728). Both tumor and normal tissue samples were obtained from Tumor Bank of Iran, Cancer Institute, Imam Khomeini Hospital Complex, Tehran University of Medical Sciences, snap-frozen in liquid nitrogen immediately, and stored at -80 °C until used for RNA extraction. A histological diagnosis of gastric cancer was an inclusion criterion.
RNA extraction and cDNA synthesis
Total RNA was extracted using the RiboEX (Genall, South Korea) RNA extraction kit. DNase1 (Sinaclon, Iran) was used to eliminate DNA contamination. A spectrophotometer was used to estimate RNA concentration. Thermo cDNA synthesis kit was used for cDNA synthesis using 1 µg of total extracted RNA according to the manufacturer's instructions.
Real-time RT-PCR analysis
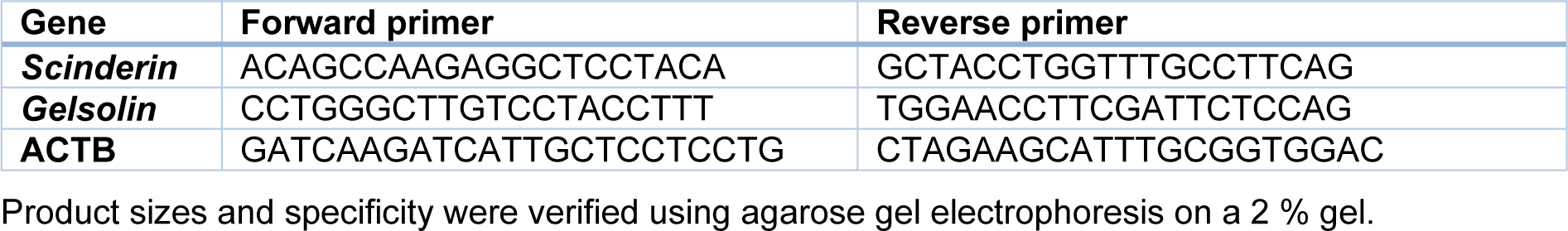
To assess the expression level of the genes of interest, quantitative real-time PCR was carried out using Corbett (Rotor-Gene 6000) and SYBR Premix Ex Taq II (Takara). For each sample, the PCR reaction reagent comprised 2 µL cDNA mixed with 1 µL forward and reverse primers and 10 µL SYBR green master mix (Takara, Japan). Then, 7 µL nuclease-free water was added to reach a final volume of 20 µL. The thermal cycling condition included an initial denaturation step at 95 ºC for 15 min, 40 cycles at 95 ºC for 15 s, at 60 ºC for 30 s, and at 72 ºC for 20 s. All reactions were done in triplicate. The data are presented as the fold change in gene expression using the 2-ΔΔCT method and normalized to the ACTB gene. The specific primer sequences for genes included in this study were shown in Table 1(Tab. 1).
Statistical analysis
SPSS 16.0 (SPSS Inc. Chicago, USA) was used for data analysis. Data distribution was evaluated using the one-sample Kolmogorov-Smirnov test. Independent sample t-test was applied to compare the differences between the two groups. One-way ANOVA was used to analyze the relationships between parameters. Numerical data are presented as mean ± standard deviation (SD). P values less than 0.05 were considered significant.
Results
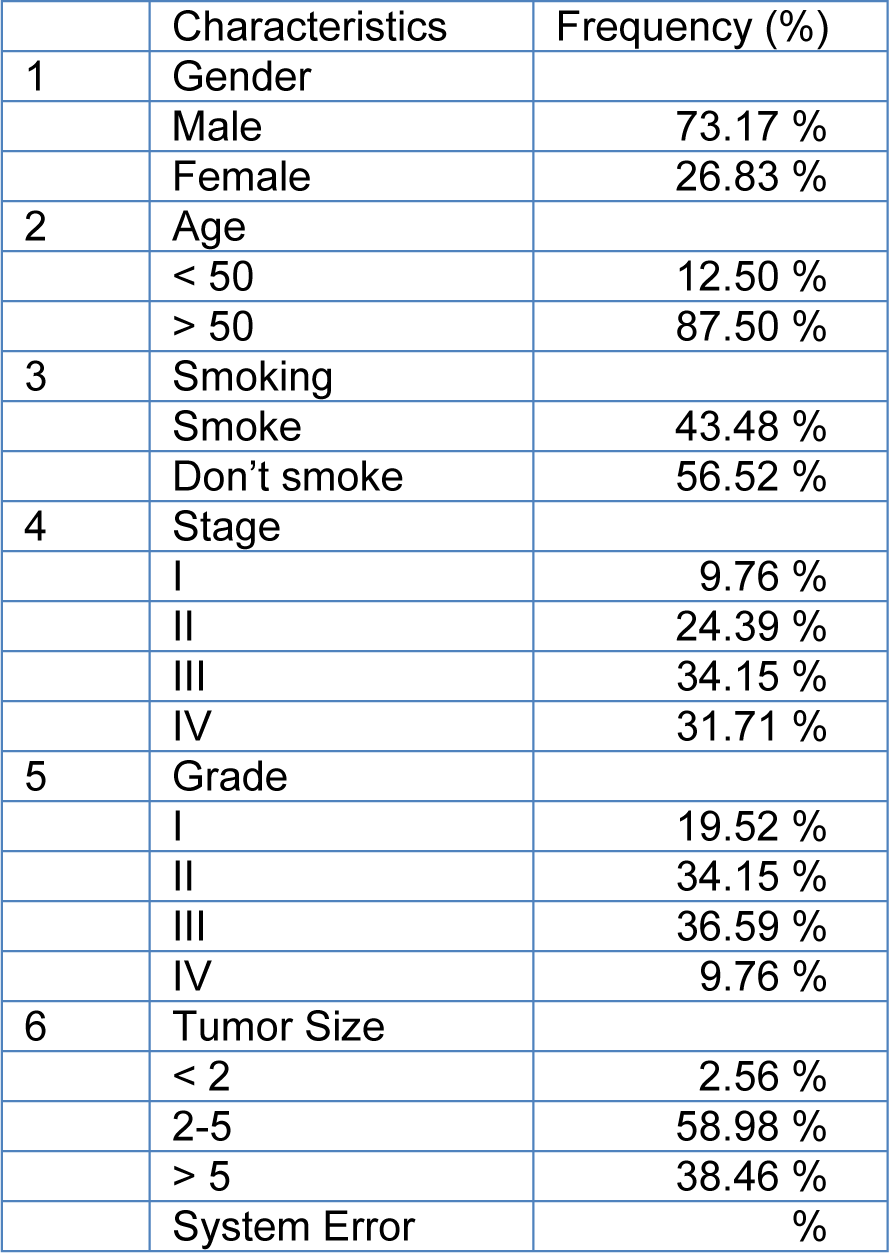
Demographic and clinical characteristics of the selected patients
Forty-five cases (mean age: 68.5) with GC stages I, II, III and IV were included in this study. Other demographic and clinical characteristics of the patients such as lymph node metastasis and tumor size are summarized in Table 2(Tab. 2). The heat maps of Scinderin and Gelsolin genes are presented in Figure 2A.
Analysis of Gelsolin and Scinderin expression levels in tumor and non-tumor samples
The results showed a significant down- regulation and up-regulation in the expression levels of Gelsolin and Scinderin in the GC tumoral tissue compared to the adjacent non-tumoral tissue (p value of 0.001), respectively. The expression of Gelsolin and Scinderin had a 2.38111 ± 0.55671 and -1.39276 ± 0.45563 fold change in GC tumor tissue samples compared to normal tissues, respectively (Figure 1A(Fig. 1)). Meanwhile, the expression of Gelsolin was significantly lower and the expression of Scinderin was significantly higher in tumor tissues compared to non-tumor tissues obtained from the same patients in this study. The results showed Gelsolin down-regulation in 87.7 % and up-regulation in 12.19 % of patients. Moreover, Scinderin was up-regulated in 85.38 % and down-regulated in 14.63 % of the patients.
Relationship between expression level of gelsolin and Scinderin and clinical features in GC patients
As the results, the expression level of Scinderin had a significant association with the patent's age (p value of 0.01). The age range of the patients with gastric cancer was 33-104 years, which was dependent on their family history and genetic background (see Table 2(Tab. 2)). A significant increase in Scinderin up-regulation and Gelsolin down-regulation was observed with an increase in age (Figure 1B(Fig. 1)).
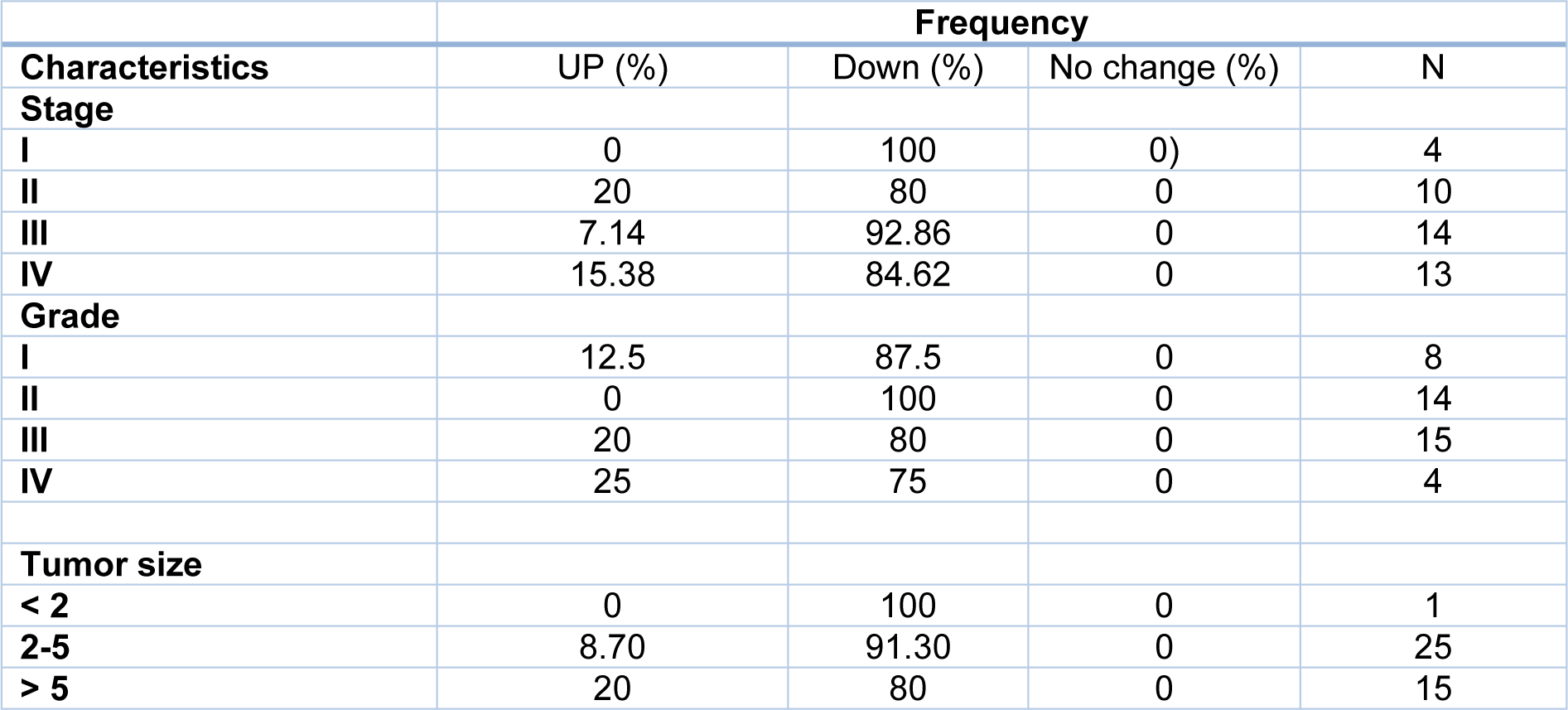
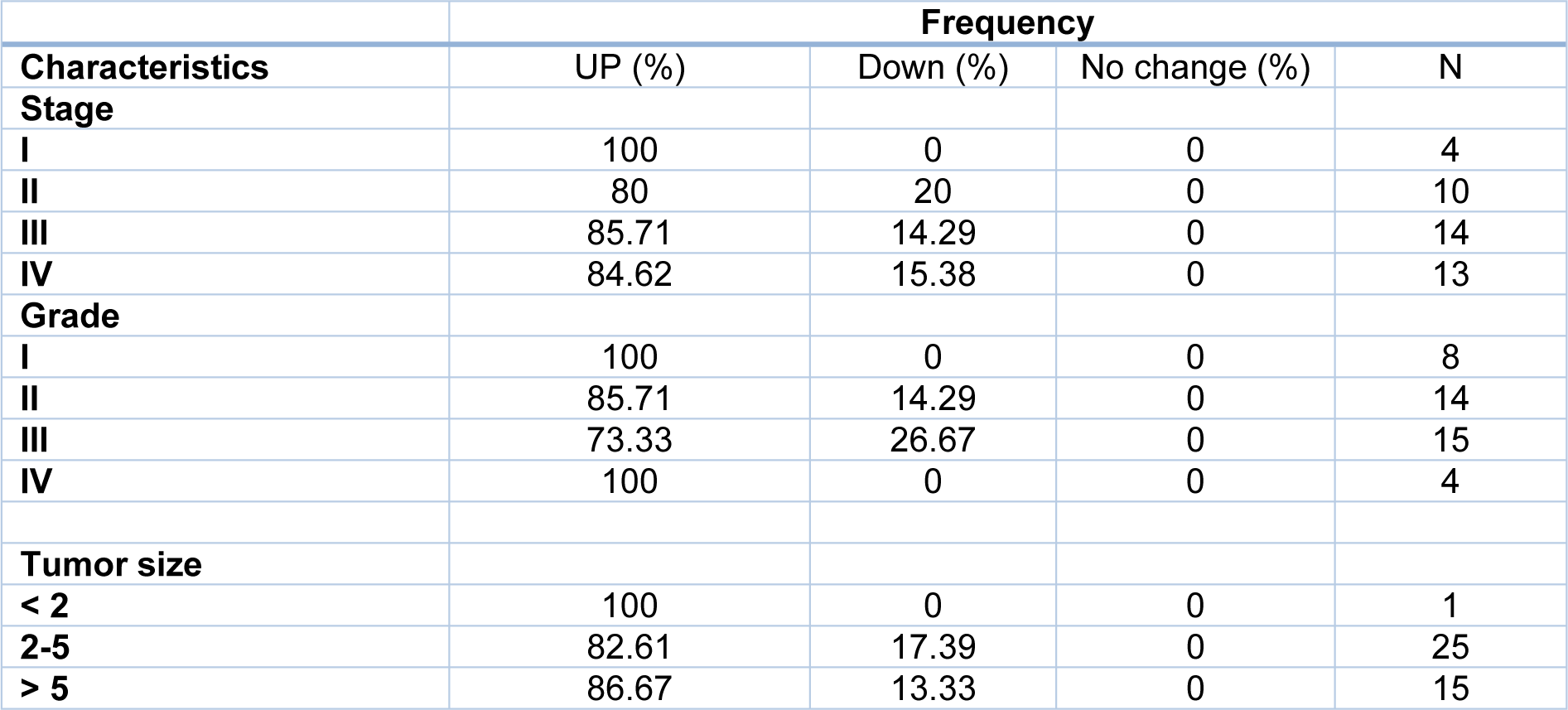
Tumor size is one of the criteria for tumor growth that is used by pathologists to determine the tumor stage and grade. In this study, tumors were classified into three groups: tumors smaller than 2 cm (T1), tumors between 2 and 5 cm (T2), and tumors larger than 5 cm (T3). According to the results of one-way ANOVA, there was no significant relationship between the Gelsolin gene expression level and tumor size while there was a significant relationship between the Scinderin expression level and tumor size (p value = 0.02) (Tables 3(Tab. 3) and 4(Tab. 4)). Based on the results, 2.56 % of the tumors were below 2 cm, 58.98 % were 2 to 5 cm, and 38.46 % of tumors were larger than 5 cm in size. Decreased expression of Gelsolin and increased expression of Scinderin were more evident in larger tumors (Figure 1C(Fig. 1)).
In this study, the patients with gastric cancer were classified by lymph node metastasis, which included 25 patients with lymph node metastasis and 16 patients without it. A correlation was seen between the level of Scinderin gene expression and lymph node metastasis, however there was no significant relationship between the level of Gelsolin gene expression and lymph node metastasis (p value= 0.005). A sharp increased expression of Scinderin and a reduction of expression of Gelsolin was more significant in samples with positive lymph node metastasis (60.97 %) in contrast with samples with no lymph node metastasis (39.02 %). There is little likelihood of a link between the expression level of these genes and lymph node metastasis (Figure 1D(Fig. 1)).
Furthermore, Gelsolin and Scinderin expression was assessed in different grades and stages to determine the association of this gene with cancer progression. The result indicates no significant association between Gelsolin expression level and GC stage while Scinderin expression level was significantly different between stage I (2.30 ± 2.75) and IV (9.6 ± 6.35) (p= 0.05), II (2.91 ± 2.32) and IV (9.60 ± 6.35) (p=0.005), and III (3.42 ± 3.83) and IV (9.6 ± 6.35) (p=0.005) (Figure 1E(Fig. 1)).
Although no significant association was observed between Scinderin expression level and GC grade, the mean Gelsolin expression showed a significant difference between grade II (0.25 ± 0.26) and III (1.01 ± 1.29) (p =0.05) as well as grade I (0.25 ± 0.26) and IV (1.84 ± 3.23) (p=0.04). It is difficult to evaluate the average expression level of Gelsolin in grades II and IV and further studies are needed (p=0.06) (Figure 1F(Fig. 1)).
Evaluation of the biomarker quality for GC
ROC curve analysis was used to evaluate the sensitivity and specificity of the expression levels of these genes to differentiate GC tissues from healthy tissues. The calculated levels of the genes examined in this study indicated that Scinderin could potentially candidate as an efficient diagnostic biomarker for GC (Figure 2B(Fig. 2)).
Discussion
Gastric cancer is the third leading cause of cancer-related death in spite of recent advances in diagnostic and therapeutic techniques. Therefore, identification of the molecular mechanisms underlying the disease has drawn the attention of many researchers since it might help to improve cancer therapy and diagnosis (Lazăr et al., 2016[22]; Torre et al., 2015[46]).
Irregular epithelial cell growth and angiogenesis are the first signs of early epithelial cancer (Kalluri and Weinberg, 2009[18]). This process is responsible for the death of more than 90 % of patients with cancer. In many studies, the EMT process has been mentioned as the main mechanism for the formation of malignant phenotypes of epithelial cancer cells (Guo and Giancotti, 2004[15]).
In addition, more evidence suggests that alterations in polymerization or actin remodeling play a key role in regulating phenotypic and morphological events in malignant cells (Thiery and Chopin, 1999[44]). Cancer cell migration can be induced by regulation of the cytoskeleton, since Actin-binding proteins have been investigated in cancer trial (Zhang and Tong, 2010[55]). The expression levels of two important actin-binding proteins, Gelsolin and Scinderin, were studied in gastric tumor and the adjacent normal tissue in this study.
Scinderin may regulate the EMT process to affect cell migration. Chen et al. (2014[5]) reported that Scinderin suppression correlated with the proliferation and migration of gastric cancer SGC-7901 cells and attenuated their EMT process. Also, Gelsolin is down-regulated in human gastric cancer; moreover, Gelsolin significantly correlates with gastric cancer metastasis. Migration of gastric cancer cells is suppressed by Gelsolin, which decreases EMT by inducing cytoskeleton remolding via inhibition of p38 signaling (Yuan et al., 2016[54]). All the mentioned studies confirmed the critical role of Scinderin and Gelsolin in tumorigenesis.
It has been shown that the closest homologue of Scinderin is involved in the cell death process in zebrafish. Wang et al. (2014[50]) found Scinderin over-expression in prostate tumor. Thus, in order to address the function of Scinderin in prostate carcinoma cells, its expression was knocked down in the PC3 cell, a prostate carcinoma cell line, using lentivirus-mediated RNAi. Reduced Scinderin expression leads to G0/G1 cell cycle arrest through cell cycle-related genes. Moreover, Scinderin silencing inhibits the proliferation and colony formation ability of PC3 cells. Liu et al. (2015[28]) found that knocking down Scinderin expression in A549 and H1299 cell lines by lentivirus-mediated RNAi was associated with similar results, including proliferation inhibition and G0/G1 cell cycle arrest. In addition, Wu et al. (2013[52]) reported a significantly lower expression of Scinderin in normal ovarian tissue and benign ovarian epithelial tumors versus ovarian epithelial cancer. However, no significant difference was observed in Scinderin expression between normal ovarian tissue and benign ovarian epithelial tumor. In another immunohistochemical study of Scinderin expression, Hasmim et al. (2013[16]) reported the over-expression of this protein in head and neck cancer. In line with previous studies, the present study showed significant over-expression of Scinderin in gastric tumor tissues compared to the adjacent normal tissue.
Knock out of Gelsolin expression as a tumor suppressor gene has been observed in at least three animal species and a variety of etiologies. Dong et al. (2002[9]) showed that reduced expression of Gelsolin was related to epigenetic factors and was not due to mutation. Furthermore, Ahn et al. (2003[2]) found that decreased Gelsolin expression was one of the most common molecular defects in breast carcinoma. In addition, according to a study by Gay et al. (2008[11]) gelsolin down-regulation is an early and constant event in clonal carcinogenesis and can cause adenoma to carcinoma, which confirms previous observations. Liu et al. (2007[29]) reported that concurrent Gelsolin and Egr-1 down-regulation might be an important marker for breast cancer. Furthermore, in line with Liu et al. (2007[29]), Winston et al. (2001[51]) also found that Gelsolin down-regulation correlated with breast carcinoma progression and might be considered as a diagnostic biomarker for this disease. Similarly, Tanaka et al. (2006[43]) suggested the Gelsolin controlled E- and N-cadherin conversion; they demonstrated that Gelsolin knock-down led to epithelial-mesenchymal transition in human mammary epithelial cells and might be involved in human mammary tumors development. Shirkoohi et al. (2012[39]) found that monocytic differentiation could be induced in U937 leukemia cells by Gelsolin through activation of p21CIP1. Evidence also suggests that up-regulation of Gelsolin can inhibit proliferation of colon, bladder, and lung cancer cells (Sagawa et al., 2003[36]; Tsai et al., 2012[49]). Thor et al. (2001[45]) reported that Gelsolin was a negative prognostic factor and motility effectors in erbB-2 positive and EGFR positive breast cancer cases. Crowley et al. (2000[6]) found that Gelsolin played a key role in ductal morphogenesis in the mouse mammary gland. According to the results of a study by Deng et al. (2015[7]) proliferation, apoptosis, migration, and invasion of human oral carcinoma cells can be regulated by Gelsolin over-expression. Contradictory results were observed in the current research since suppression of Gelsolin expression correlated with cancer onset, progression, and invasion.
The results of this study showed no significant association between GC stage and Gelsolin expression level. In spite of these results, Yuan et al. (2016[54]) found that Gelsolin expression was down-regulated in advanced stages of gastric cancer, including III and IV, in comparison to earlier stages, i.e. I and II. On the other hand, Tsai et al. (2012[49]) reported Gelsolin over-expression in late stages of gastric cancer compared to earlier stages. This difference might be due to the limited number of samples in their study. Thus, in order to obtain a more confident conclusion, more extensive studies with larger sample sizes are required. In addition, Gelsolin and Scinderin expression levels showed a significant association with GC stage. As mentioned earlier, Liu et al. (2016[30]) found no significant association between Scinderin expression level and GC grade. However, evidence suggests a significant association between Scinderin expression level and ovarian cancer stage (Wu et al., 2013[52]). There was a significant association between Gelsolin expression level and GC grade in grades I and II. Nevertheless, more extensive studies with larger sample sizes are needed to assess any possible association between the expression level of this gene and tumor grade in grades II and IV. Winston et al. (2001[51]) found a significant reduction in Gelsolin expression level in advanced grades of breast cancer and Yamaguchi and Condeelis (2007[53]) reported a significant association between Gelsolin expression level and tumor grade. Liu et al. (2016[30]) found that tumors involving Scinderin repression were significantly smaller compared to the tumors expressing this gene. Similar results were also obtained in this study, and a significant association was observed between tumor size and Scinderin expression level. In contrast to Scinderin, no signification association was found between Gelsolin expression level and tumor size. Li et al. (2009[26]) also reported no significant association between Gelsolin expression level and tumor size. A significant increase was observed in Scinderin expression in patients above 40 years. Nonetheless, Wu et al. (2013[52]) found no significant relationship between Scinderin expression and patient's age. The results of the present study showed decreased Gelsolin expression in patients above 40 years, while the relationship between Gelsolin expression and age was not significant. In two other studies, Winston et al. (2001[51]) and Abedini et al. (2014[1]) also found no significant relationship between Gelsolin expression and patient's age. Lymph node metastasis was observed in 59.52 % of the cases included in this study. The results showed that reduced Gelsolin and increased Scinderin expression were related to lymph node metastasis. However, in order to elucidate their role in lymph node metastasis, more research is warranted into the regulatory pathways of these genes.
Conclusion
Scinderin and Gelsolin genes play key roles in gastric cancer initiation, progression, and invasion. According to the result of this study, their expression level significantly correlates with GC. Overall, more extensive in vitro and in vivo studies with larger sample sizes are warranted to deepen our understanding of the roles of Gelsolin and Scinderin genes. Moreover, assessment of Gelsolin and Scinderin expression at the protein level might also be helpful. So, further studies would be promising to find new more efficient biomarkers.
Conflict of interest
The authors declare that they have no conflict of interests.
Acknowledgements
We humbly thank Dr Babak Saedi from Tehran University for his help in editing this article.
References
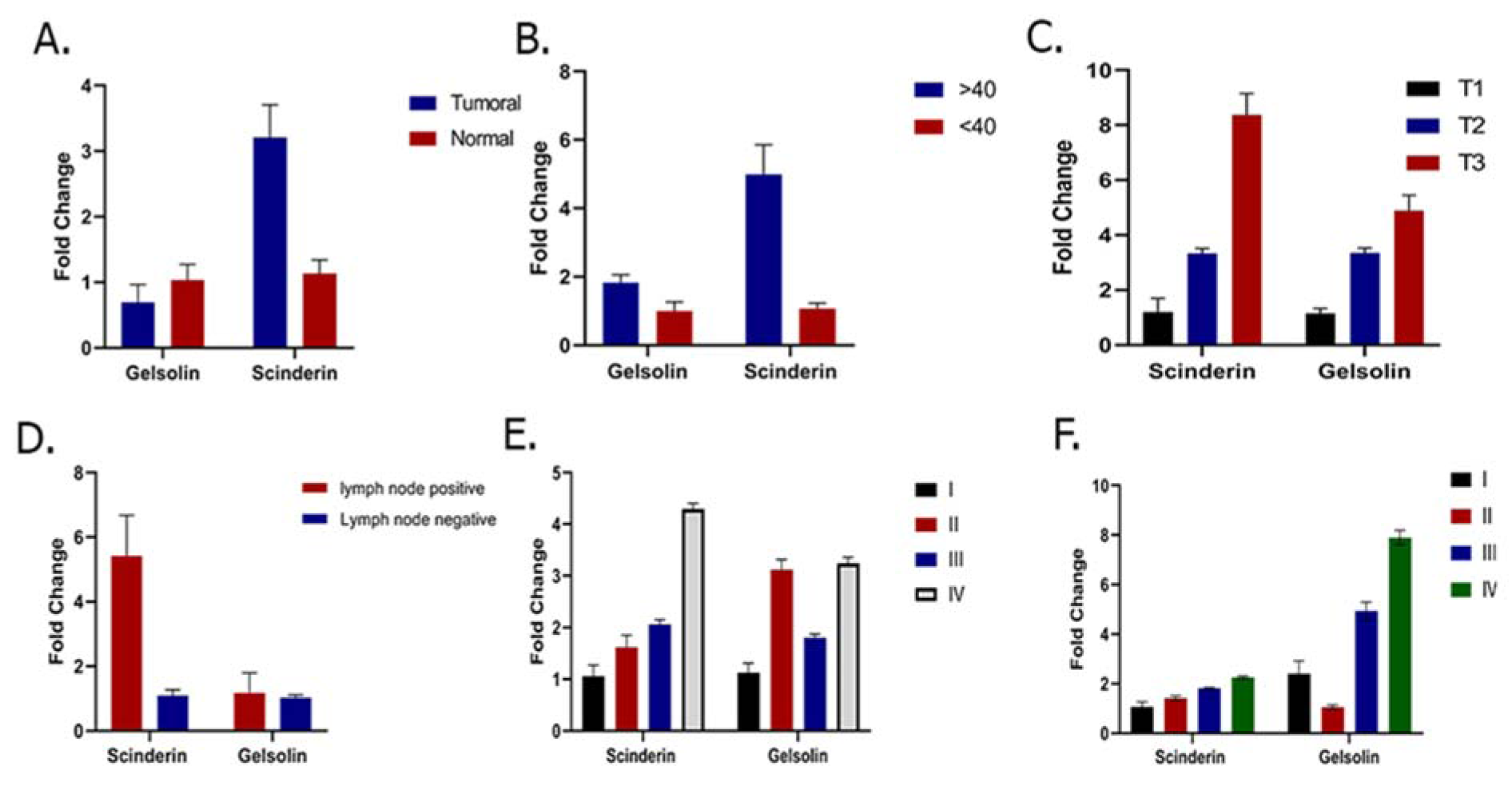
Figure 1: A. Comparison of Gelsolin and Scinderin expression level in GC patients: this figure demonstrates significant down-regulation of Gelsolin and up-regulation of Scinderin expression levels in GC in comparison with adjacent normal tissue (p = 0.001). B. Relationship between Scinderin and Gelsolin expression and patient's age. Scinderin expression level was significantly associated with age (p= 0.01). C. Relationship between Scinderin and Gelsolin and tumor size: D. Relationship between Scinderin and gelsolin expression and lymph node metastasis. E. Relationship between Scinderin and Gelsolin expression and different stages of GC. F. Relationship between Scinderin and Gelsolin expression and different grades of GC.
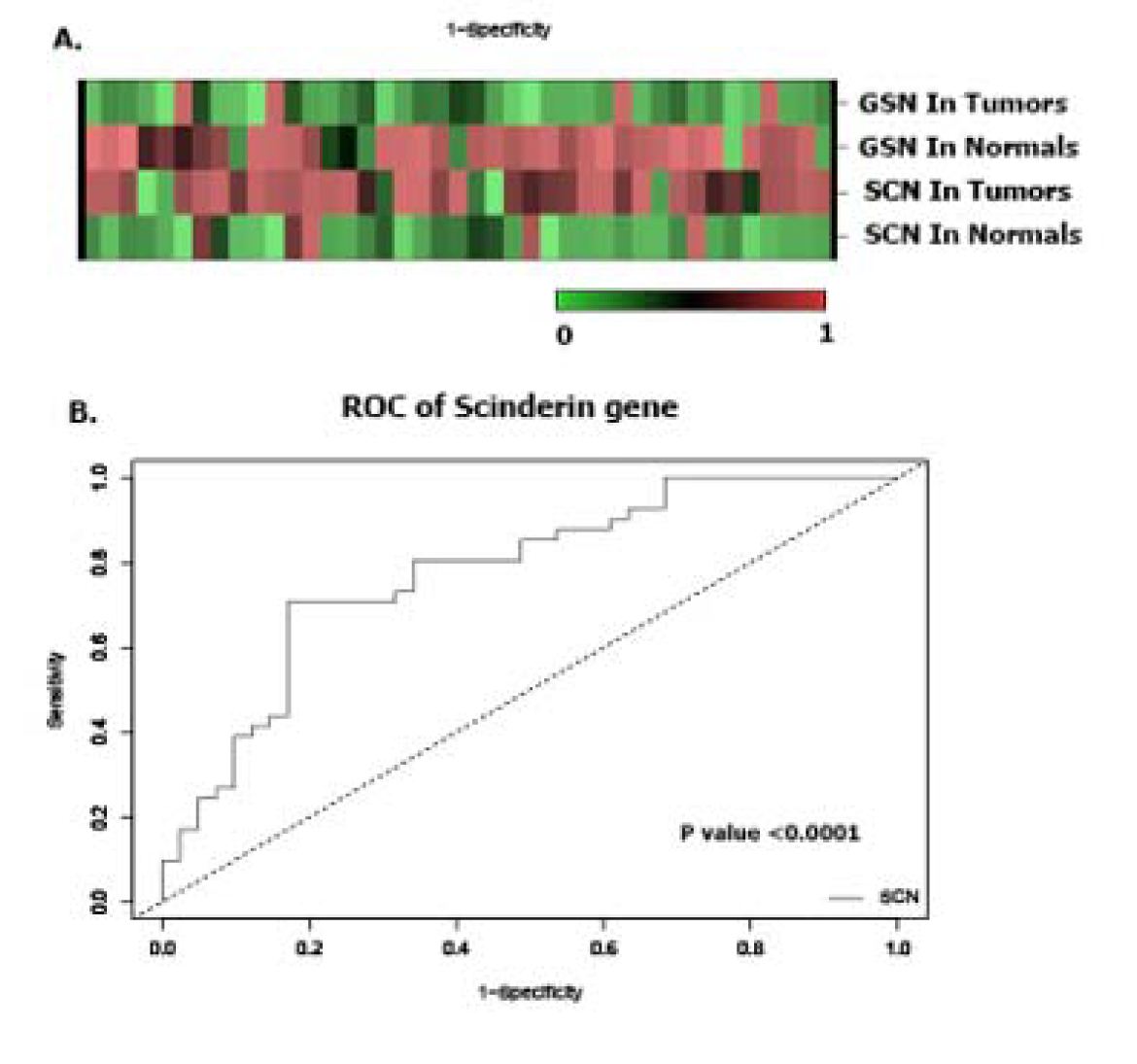
Figure 2: A. The heatmap (Euclidian distance, complete linkage) of Scinderin and Gelsolin genes. This figure shows high expression of Scinderin and gelsolin in green (GC) and their low expression in red (normal tissues). B. ROC curve analysis was done to differentiate tumor from non-tumor tissue samples. ROC curve and its calculated area under curve (AUC) of Scinderin showed that it might be a suitable tumor biomarker for GC.
[*] Corresponding Author:
Reza Shirkoohi, Cancer Research Institute, Tehran University of Medical Sciences, Tehran, Iran; Tel: +989126807836, eMail: rshirkoohi@tums.ac.ir