Letter to the editor
Recent studies on pinene and its biological and pharmacological activities
Byung Bae Park1, Ji Young An1, Sang Un Park2
1Department of Environment and Forest Resources, Chungnam National University, 99 Daehak-ro, Yuseong-gu, Daejeon 34134, Korea
2Department of Crop Science and Department of Smart Agriculture Systems, Chungnam National University, 99 Daehak-ro, Yuseong-gu, Daejeon 34134, Korea
EXCLI J 2021;20:Doc812
Dear Editor,
Pinene (C10H16) is a well-known group of monoterpenes and the main component of turpentine, which is a fluid obtained by the distillation of resin harvested from coniferous trees, particularly those of the genus Pinus (Mercier et al., 2009[31]; Al-Tel et al., 2020[1]). Pinene can be divided into two structural isomers: α-pinene (α-pinene) and beta-pinene (β-pinene). α- and β-pinene are mainly produced by pine trees and many other conifers, as well as a wide range of herbs such as rosemary, parsley, basil, and even orange peel (Erman and Kane, 2008[9]; Vespermann et al., 2017[45]).
α-pinene, the most abundant monoterpene in the atmosphere, accounts for more than 50 % of global monoterpene emissions and is a major component of phytoncides (Bagchi et al., 2020[2]; Li et al., 2009[27]). Phytoncides are antimicrobial allelochemical volatile organic compounds that are related to forest healing and activation of recreational forests. Trees are considered one of the major emitters of phytoncides (Li, 2010[26]).
A wide range of pharmacological activities of α- and β-pinene have been reported, such as anticoagulant, anti-inflammatory, anti-leishmania, antimalarial, antimicrobial, antioxidant, antitumor, analgesic, and antibiotic resistance modulation effects (Türkez and Aydın, 2016[42]; Salehi et al., 2019[40]). These monoterpenes exhibit various biological activities and have a wide range of applications, including development of antimicrobial and antiviral agents, flavors, fragrances, and fungicidal agents (Rivas da Silva et al., 2012[38]; Yang et al., 2013[49]). Herein, we summarize the recent published findings on the biological and pharmacological activities of pinene (Table 1(Tab. 1); References in Table 1: Bouzenna et al., 2017[3]; Cardoso et al., 2020[4]; Chen et al., 2015[5]; de Macêdo et al., 2018[6]; do Amaral et al., 2020[7]; Ensaka and Sakamoto, 2020[8]; Felipe et al., 2019[10]; Govindarajan et al., 2016[11]; Hou et al., 2019[12]; İnce et al., 2018[13]; Jensen et al., 2020[14]; Jo et al., 2021[15]; Karthikeyan et al., 2018[17], 2019[16]; Kasuya et al., 2015[18]; Khoshnazar et al., 2019[19], 2020[20]; Kim et al., 2015[21]; Kovač et al., 2015[22]; Kusuhara et al., 2019[23]; Langsi et al., 2020[24]; Lee et al., 2017[25]; Li et al., 2016[28]; Memariani et al., 2017[29]; Meng et al., 2020[30]; Min et al., 2020[32]; Moreira et al., 2016[33]; Nóbrega et al., 2020[34]; Pajaro-Castro et al., 2017[35]; Pinheiro et al., 2015[36]; Porres-Martínez et al., 2016[37]; Rodrigues et al., 2015[39]; Šimunović et al., 2020[41]; Ueno et al., 2019[44], 2020[43]; Wang et al., 2019[46]; Xu et al., 2018[47]; Yang et al., 2016[48]; Zamyad et al., 2019[50]; Zhang et al., 2015[52], 202[51]0; Zhao et al., 2018[53]).
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2021R1A2C201017811) and this study was carried out with the support of ‘R&D Program for Forest Science Technology (Project No. 2021379B10-2123-BD02)’ provided by Korea Forest Service (Korea Forestry Promotion Institute).
Conflict of interest
The authors declare no conflict of interest.
References
1.
Al-Tel TH, Tarazi H, Aloum LO, Lorke DE, Petroianu GA. Possible metabolic conversion of pinene to ionone. Pharmazie. 2020;75:360-3.2.
Bagchi A, Yu Y, Huang JH, Tsai CC, Hu WP, Wang CC. Evidence and evolution of Criegee intermediates, hydroperoxides and secondary organic aerosols formed via ozonolysis of α-pinene. Phys Chem Chem Phys. 2020;22:6528-37.3.
Bouzenna H, Hfaiedh N, Giroux-Metges MA, Elfeki A, Talarmin H. Potential protective effects of alpha-pinene against cytotoxicity caused by aspirin in the IEC-6 cells. Biomed Pharmacother. 2017;93:961-8. 4.
Cardoso P, Nunes T, Pinto R, Sá C, Matos D, Figueira E. Rhizobium response to sole and combined exposure to cadmium and the phytocompounds alpha-pinene and quercetin. Ecotoxicology. 2020;29:444-58. 5.
Chen W, Liu Y, Li M, Mao J, Zhang L, Huang R, et al. Anti-tumor effect of α-pinene on human hepatoma cell lines through inducing G2/M cell cycle arrest. J Pharmacol Sci. 2015;127:332-8.6.
de Macêdo Andrade AC, Rosalen PL, Freires IA, Scotti L, Scotti MT, Aquino SG, de Castro RD. Antifungal activity, mode of action, docking prediction and anti-biofilm effects of (+)-β-pinene enantiomers against Candida spp. Curr Top Med Chem. 2018;18:2481-90.7.
do Amaral FLE, Farias TC, de Brito RC, de Melo TR, Ferreira PB, Lima ZN, e al. Effect of the association and evaluation of the induction to adaptation of the (+)-α-pinene with commercial antimicrobials against strains of Escherichia coli. Curr Top Med Chem. 2020;20:2300-7. 8.
Ensaka N, Sakamoto K. α-Pinene odor exposure enhances heat stress tolerance through Daf-16 in Caenorhabditis elegans. Biochem Biophys Res Commun. 2020;528:726-31. 9.
Erman MB, Kane BJ. Chemistry around pinene and pinane: a facile synthesis of cyclobutanes and oxatricyclo-derivative of pinane from cis- and trans-pinanols. Chem Biodivers. 2008;5:910-9.10.
Felipe CFB, Albuquerque AMS, de Pontes JLX, de Melo JÍV, Rodrigues TCML, de Sousa AMP, et al. Comparative study of alpha- and beta-pinene effect on PTZ-induced convulsions in mice. Fundam Clin Pharmacol. 2019;33:181-90. 11.
Govindarajan M, Rajeswary M, Hoti SL, Bhattacharyya A, Benelli G. Eugenol, α-pinene and β-caryophyllene from Plectranthus barbatus essential oil as eco-friendly larvicides against malaria, dengue and Japanese encephalitis mosquito vectors. Parasitol Res. 2016;115:807-15. 12.
Hou J, Zhang Y, Zhu Y, Zhou B, Ren C, Liang S, et al. α-Pinene induces apoptotic cell death via caspase activation in human ovarian cancer cells. Med Sci Monit. 2019;25:6631-8. 13.
İnce B, Dadacı M, Kılınç İ, Oltulu P, Yarar S, Uyar M. Effect of cineole, alpha-pinene, and camphor on survivability of skin flaps. Turk J Med Sci. 2018;48:644-52.14.
Jensen TG, Holmstrup M, Madsen RB, Glasius M, Trac LN, Mayer P, et al. Effects of α-pinene on life history traits and stress tolerance in the springtail Folsomia candida. Comp Biochem Physiol C Toxicol Pharmacol. 2020;229:108681. 15.
Jo H, Cha B, Kim H, Brito S, Kwak BM, Kim ST, et al. α-pinene enhances the anticancer activity of natural killer cells via ERK/AKT pathway. Int J Mol Sci. 2021;22:656. 16.
Karthikeyan R, Kanimozhi G, Madahavan NR, Agilan B, Ganesan M, Prasad NR, et al. Alpha-pinene attenuates UVA-induced photoaging through inhibition of matrix metalloproteinases expression in mouse skin. Life Sci. 2019;217:110-8. 17.
Karthikeyan R, Kanimozhi G, Prasad NR, Agilan B, Ganesan M, Srithar G. Alpha pinene modulates UVA-induced oxidative stress, DNA damage and apoptosis in human skin epidermal keratinocytes. Life Sci. 2018;212:150-8. 18.
Kasuya H, Okada N, Kubohara M, Satou T, Masuo Y, Koike K. Expression of BDNF and TH mRNA in the brain following inhaled administration of α-pinene. Phytother Res. 2015;29:43-7. 19.
Khoshnazar M, Bigdeli MR, Parvardeh S, Pouriran R. Attenuating effect of α-pinene on neurobehavioural deficit, oxidative damage and inflammatory response following focal ischaemic stroke in rat. J Pharm Pharmacol. 2019;71:1725-33. 20.
Khoshnazar M, Parvardeh S, Bigdeli MR. Alpha-pinene exerts neuroprotective effects via anti-inflammatory and anti-apoptotic mechanisms in a rat model of focal cerebral ischemia-reperfusion. J Stroke Cerebrovasc Dis. 2020;29:104977. 21.
Kim DS, Lee HJ, Jeon YD, Han YH, Kee JY, Kim HJ, et al. Alpha-pinene exhibits anti-inflammatory activity through the suppression of MAPKs and the NF-κB pathway in mouse peritoneal macrophages. Am J Chin Med. 2015;43:731-42. 22.
Kovač J, Šimunović K, Wu Z, Klančnik A, Bucar F, Zhang Q, et al. Antibiotic resistance modulation and modes of action of (-)-α-pinene in Campylobacter jejuni. PLoS One. 2015;10:e0122871. 23.
Kusuhara M, Maruyama K, Ishii H, Masuda Y, Sakurai K, Tamai E, et al. A fragrant environment containing α-pinene suppresses tumor growth in mice by modulating the hypothalamus/sympathetic nerve/leptin axis and immune system. Integr Cancer Ther. 2019;18:1534735419845139. 24.
Langsi JD, Nukenine EN, Oumarou KM, Moktar H, Fokunang CN, Mbata GN. Evaluation of the insecticidal activities of α-pinene and 3-carene on Sitophilus zeamais motschulsky (Coleoptera: Curculionidae). Insects. 2020;11:540.25.
Lee GY, Lee C, Park GH, Jang JH. Amelioration of scopolamine-induced learning and memory impairment by α-pinene in C57BL/6 mice. Evid Based Complement Alternat Med. 2017;2017:4926815. 26.
Li Q. Effect of forest bathing trips on human immune function. Environ Health Prev Med. 2010;15:9-17.27.
Li Q, Kobayashi M, Wakayama Y, Inagaki H, Katsumata M, Hirata Y, et al. Effect of phytoncide from trees on human natural killer cell function. Int J Immunopathol Pharmacol. 2009;22:951-9. 28.
Li XJ, Yang YJ, Li YS, Zhang WK, Tang HB. α-Pinene, linalool, and 1-octanol contribute to the topical anti-inflammatory and analgesic activities of frankincense by inhibiting COX-2. J Ethnopharmacol. 2016;179:22-6. 29.
Memariani Z, Sharifzadeh M, Bozorgi M, Hajimahmoodi M, Farzaei MH, Gholami M, et al. Protective effect of essential oil of Pistacia atlantica Desf. on peptic ulcer: Role of α-pinene. J Tradit Chin Med. 2017;37:57-63. 30.
Meng F, Li Y, Liu Z, Wang X, Feng Y, Zhang W, et al. Potential molecular mimicry proteins responsive to α-pinene in Bursaphelenchus xylophilus. Int J Mol Sci. 2020;21:982.31.
Mercier B, Prost J, Prost M. The essential oil of turpentine and its major volatile fraction (alpha- and beta-pinenes): A review. Int J Occup Med Environ Health. 2009;22:331-42.32.
Min HY, Son HE, Jang WG. Alpha-pinene promotes osteoblast differentiation and attenuates TNFα-induced inhibition of differentiation in MC3T3-E1 pre-osteoblasts. Clin Exp Pharmacol Physiol. 2020;47:831-7. 33.
Moreira IJ, Menezes PP, Serafini MR, Araújo AA, Quintans-Júnior LJ, Bonjardim LR, et al. Characterization and antihypertensive effect of the complex of (-)-β- pinene in β-cyclodextrin. Curr Pharm Biotechnol. 2016;17:837-45.34.
Nóbrega JR, Silva DF, Andrade Júnior FP, Sousa PMS, Figueiredo PTR, Cordeiro LV, et al. Antifungal action of α-pinene against Candida spp. isolated from patients with otomycosis and effects of its association with boric acid. Nat Prod Res. 2020;23:1-4. 35.
Pajaro-Castro N, Caballero-Gallardo K, Olivero-Verbel J. Neurotoxic effects of linalool and β-pinene on Tribolium castaneum Herbst. Molecules. 2017;22:2052. 36.
Pinheiro MDA, Magalhães RM, Torres DM, Cavalcante RC, Mota FS, Oliveira Coelho EM, et al. Gastroprotective effect of alpha-pinene and its correlation with antiulcerogenic activity of essential oils obtained from Hyptis species. Pharmacogn Mag. 2015;11:123-30. 37.
Porres-Martínez M, González-Burgos E, Carretero ME, Gómez-Serranillos MP. In vitro neuroprotective potential of the monoterpenes α-pinene and 1,8-cineole against H2O2-induced oxidative stress in PC12 cells. Z Naturforsch C J Biosci. 2016;71:191-9.38.
Rivas da Silva AC, Lopes PM, Barros de Azevedo MM, Costa DC, Alviano CS, Alviano DS. Biological activities of α-pinene and β-pinene enantiomers. Molecules. 2012;17:6305-16. 39.
Rodrigues KA, Amorim LV, Dias CN, Moraes DF, Carneiro SM, Carvalho FA. Syzygium cumini (L.) Skeels essential oil and its major constituent α-pinene exhibit anti-Leishmania activity through immunomodulation in vitro. J Ethnopharmacol. 2015;160:32-40. 40.
Salehi B, Upadhyay S, Erdogan Orhan I, Kumar Jugran A, Jayaweera SLD, Dias DA, et al. Therapeutic potential of α- and β-pinene: A miracle gift of nature. Biomolecules. 2019;9:738. 41.
Šimunović K, Sahin O, Kovač J, Shen Z, Klančnik A, Zhang Q, et al. (-)-α-Pinene reduces quorum sensing and Campylobacter jejuni colonization in broiler chickens. PLoS One. 2020;15:e0230423. 42.
Türkez H, Aydın E. In vitro assessment of cytogenetic and oxidative effects of α-pinene. Toxicol Ind Health. 2016;32:168-76.43.
Ueno H, Shimada A, Suemitsu S, Murakami S, Kitamura N, Wani K, et al. Alpha-pinene and dizocilpine (MK-801) attenuate kindling development and astrocytosis in an experimental mouse model of epilepsy. IBRO Rep. 2020;9:102-14. 44.
Ueno H, Shimada A, Suemitsu S, Murakami S, Kitamura N, Wani K, et al. Attenuation effects of alpha-pinene inhalation on mice with dizocilpine-induced psychiatric-like behaviour. Evid Based Complement Alternat Med. 2019:2745453. 45.
Vespermann KA, Paulino BN, Barcelos MC, Pessôa MG, Pastore GM, Molina G. Biotransformation of α- and β-pinene into flavor compounds. Appl Microbiol Biotechnol. 2017;101:1805-17.46.
Wang X, Yu Y, Ge J, Xie B, Zhu S, Cheng X. Effects of α-pinene on the pinewood nematode (Bursaphelenchus xylophilus) and its symbiotic bacteria. PLoS One. 2019;14:e0221099.47.
Xu Q, Li M, Yang M, Yang J, Xie J, Lu X, et al. α-pinene regulates miR-221 and induces G2/M phase cell cycle arrest in human hepatocellular carcinoma cells. Biosci Rep. 2018;38:BSR20180980. 48.
Yang H, Woo J, Pae AN, Um MY, Cho NC, Park KD, et al. α-pinene, a major constituent of pine tree oils, enhances non-rapid eye movement sleep in mice through GABAA-benzodiazepine receptors. Mol Pharmacol. 2016;90:530-9. 49.
Yang J, Nie Q, Ren M, Feng H, Jiang X, Zheng Y, et al. Metabolic engineering of Escherichia coli for the biosynthesis of alpha-pinene. Biotechnol Biofuels. 2013;6:60.50.
Zamyad M, Abbasnejad M, Esmaeili-Mahani S, Mostafavi A, Sheibani V. The anticonvulsant effects of Ducrosia anethifolia (Boiss) essential oil are produced by its main component alpha-pinene in rats. Arq Neuropsiquiatr. 2019;77:106-14. 51.
Zhang B, Wang H, Yang Z, Cao M, Wang K, Wang G, et al. Protective effect of alpha-pinene against isoproterenol-induced myocardial infarction through NF-κB signaling pathway. Hum Exp Toxicol. 2020;39:1596-606. 52.
Zhang Z, Guo S, Liu X, Gao X. Synergistic antitumor effect of α-pinene and β-pinene with paclitaxel against non-small-cell lung carcinoma (NSCLC). Drug Res (Stuttg). 2015;65:214-8. 53.
Zhao Y, Chen R, Wang Y, Yang Y. α-pinene inhibits human prostate cancer growth in a mouse xenograft model. Chemotherapy. 2018;63:1-7.
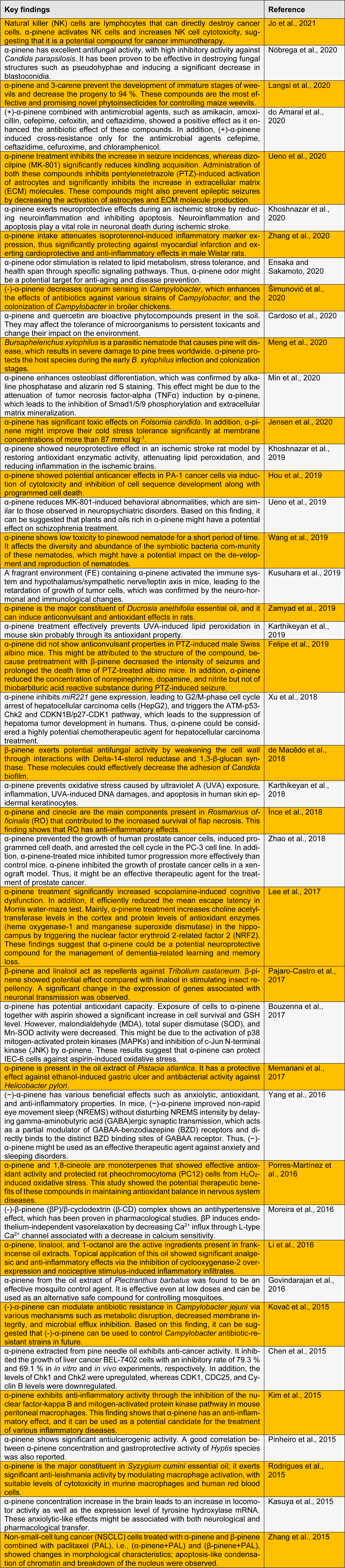
Table 1: Recent studies on the biological and pharmacological activities of pinene
