Review article
Inconsistent effects of sleep deprivation on memory function
Salar Vaseghi1[*],2, Shirin Arjmandi-Rad3, Gita Kholghi1, Mohammad Nasehi1
1Cognitive and Neuroscience Research Center (CNRC), Amir-Almomenin Hospital, Tehran Medical Sciences, Islamic Azad University, Tehran, Iran2Department of Cognitive Neuroscience, Institute for Cognitive Science Studies (ICSS), Tehran, Iran
3Institute for Cognitive & Brain Sciences, Shahid Beheshti University, Tehran, Iran
EXCLI J 2021;20:Doc1011
Abstract
In this review article, we aimed to discuss the role of sleep deprivation (SD) in learning and memory processing in basic and clinical studies. There are numerous studies investigating the effect of SD on memory, while most of these studies have shown the impairment effect of SD. However, some of these studies have reported conflicting results, indicating that SD does not impair memory performance or even improves it. So far, no study has discussed or compared the conflicting results of SD on learning and memory. Thus, this important issue in the neuroscience of sleep remains unknown. The main goal of this review article is to compare the similar mechanisms between the impairment and the improvement effects of SD on learning and memory, probably leading to a scientific solution that justifies these conflicting results. We focused on the inconsistent effects of SD on some mechanisms involved in learning and memory, and tried to discuss the inconsistent effects of SD on learning and memory.
Keywords: sleep deprivation, memory, learning, beneficial, destructive
Introduction
In today's world, sleep deprivation (SD) is widespread. Unfortunately, the modern life restricts the essential time needed for sleep. There are many factors involved in SD including drinking alcohol, consuming caffeine, shift work, excessive light and noise, stress, anxiety, rumination, and some diseases (Medic et al., 2017[68]). Furthermore, sleep disorders including insomnia, narcolepsy, restless leg syndrome, and obstructive sleep apnea can lead to SD (Medic et al., 2017[68]). As we know, SD induces a wide-range of negative effects on cognitive functions (Rezaie et al., 2020[91][92]). Previous studies have shown that memory and mood state are highly affected following SD (Javad-Moosavi et al., 2020[45]; Kordestani-Moghadam et al., 2020[52]; Mahdavi et al., 2020[65]). But why do sleep restrictions dramatically disrupt many of the cognitive and physiological processes? To answer this question, we can point to a neurobiological perspective of SD (Krause et al., 2017[55]): It is very important to characterize which brain networks are vulnerable or resilient to the effects of SD and to understand how SD-induced alterations (changes in the activity of different brain areas or their connectivity) justify the maladaptive changes in cognitive functions related to SD. SD induces selective effects on certain brain structures and functions, while there are individual differences in the effects of SD (Alhola and Polo-Kantola, 2007[4]). As mentioned, SD strongly disrupts memory processing. But interestingly, many studies have shown conflicting results. Some studies have reported the beneficial effects of SD on learning and memory, and some studies have not shown any destructive effect. This is an important dilemma, and the main goal of the present review is to discuss these contradictory effects. We need to reach a clear position on SD and its effects on learning and memory processing. Due to the increasing prevalence of SD in our societies, there is a need to discover what really are the effects of SD on memory function? Thus, at first, we discuss the effects of SD on different types of memory in basic and clinical studies.
SD and Memory
Impairment effects of SD on learning and memory
There are many studies reporting the impairment effect of total SD (TSD) or rapid-eye movement SD (RSD) on learning and memory. In this review, we do not mention all these studies; This is a repetitive work. Thus, we declare some of these studies. Numerous studies have shown the adverse effects of SD on learning and memory (Chen et al., 2020[18]; Zhang et al., 2020[125]). It has been shown that TSD or RSD for 24 hours disrupts passive avoidance memory acquisition in rats (Eydipour et al., 2020[23]; Javad-Moosavi et al., 2020[45]). 24 hours TSD impairs memory performance in the shuttle box apparatus in rats (Rezaie et al., 2020[91]). 24 hours TSD also impairs social interaction memory in rats (Almaspour et al., 2020[5], Rezaie et al., 2020[92]). Furthermore, 48 hours TSD impairs fear-conditioning memory in rats (Kordestani-Moghadam et al., 2020[51]). 24 hours TSD or RSD also attenuates memory formation in the inhibitory passive avoidance apparatus in rats (Javad-Moosavi et al., 2017[46]). 12, or 24-, or 36-hours RSD impairs contextual memory retrieval, while 24- or 36-hours RSD impairs auditory fear memory retention (Nasehi et al., 2019[77]). Previous research has declared that sleep is essential not only for acquisition and consolidation but also for the retrieval of fear memories, indicating 24 hours TSD impairs fear memory retrieval (Montes-Rodriguez et al., 2019[71]). It has been reported that 14-day chronic SD impairs spatial memory, object location recognition memory, and novel object recognition memory in rats (Tang et al., 2020[105]). Previous research has also shown that TSD decreases working memory capacity (Peng et al., 2020[84]). RSD impairs spatial memory performance in rats (Ocalan et al., 2019[79]). 72 hours RSD induces an impairment effect on spatial learning and memory of rats in the Morris water maze apparatus (Wang et al., 2009[117]). Chronic RSD also impairs short- and long-term memory in the radial arm water maze task (Alzoubi et al., 2017[9]). Also, chronic TSD impairs short- and long-term memory in the radial arm water maze task (Alzoubi et al., 2018[8]). Brief periods of SD can disrupt consolidation of hippocampus-dependent associative and spatial learning in rodents (Smith and Rose, 1996[102]; Graves et al., 2003[32]). It has been declared that SD suppresses neurogenesis induced by hippocampus-dependent learning in rats (Hairston et al., 2005[36]). The negative interaction effects of chronic and acute SD on spatial working memory in human have been shown in a recent study (Hennecke et al., 2020[41]). It has been shown that acute SD induces weakly encoded memories in human subjects (Baena et al., 2020[11]). 24 hours SD also impairs working memory in healthy adults (Guo et al., 2019[33]). TSD for 24 hours also impairs working memory in healthy young men (Sauvet et al., 2020[95]) (Table 1(Tab. 1); References in Table 1: Almaspour et al., 2020[5]; Alzoubi et al., 2017[9], 2018[8]; Eydipour et al., 2020[23]; Guo et al., 2019[33]; Hennecke et al., 2020[41]; Javad-Moosavi et al., 2017[46], 2020[45]; Kordestani-Moghadam et al., 2020[51]; Montes-Rodriguez et al., 2019[71]; Nasehi et al., 2019[77]; Ocalan et al., 2019[79]; Rezaie et al., 2020[91][92]; Sauvet et al., 2020[95]; Tang et al., 2020[105]; Wang et al., 2009[117]).
Improvement (or no impairment) effects of SD on learning and memory
On the contrary, there are some reports showing the improvement effect of SD on learning and memory. We prefer to focus on these results, according to the goal of the present review. For example, it has been reported that 24 hours acute SD improves learning and memory in splenectomized rats (Zhang et al., 2020[127]). Short-term SD ameliorates the impairments in learning and memory in rats following global cerebral ischemia/reperfusion (Cheng et al., 2015[19]). 24 hours RSD also improves spatial memory impairment in male Wistar rats (Mahboubi et al., 2019[64]). Interestingly, a previous study has reported that short-term SD stimulates hippocampal neurogenesis in rats (Cheng et al., 2015[19]). In addition, in a previous study, 12 hours short-term SD promoted neurogenesis in the hippocampus of rats (Junek et al., 2010[48]). Another study has shown that one night SD stimulates neurogenesis in the dentate gyrus of rats via increasing cell proliferation and survival of newly generated cells, while SD has no effect on the number of newly generated cells in the subventricular zone of the lateral ventricles, suggesting a region-specific response to SD (Grassi Zucconi et al., 2006[31]). Importantly, it has been suggested that SD, less than 24 hours, may actually increase cell proliferation (Mueller et al., 2015[76]). It has been also revealed that acute RSD improves avoidance learning and spatial memory in rats (Azogu et al., 2015[10]). Post-learning paradoxical SD also enhances avoidance performance in rats (Gisquet-Verrier and Smith, 1989[30]). In addition, a previous study has shown that acquisition of memory is not affected by SD in honeybees (Hussaini et al., 2009[42]). SD during several days has no effect on long-term recall of declarative and procedural memories in adolescents (Voderholzer et al., 2011[114]). Another related study has shown that acute SD does not affect declarative memory in 10‐year‐old girls (Biggs et al., 2010[12]). Furthermore, verbal working memory and declarative memory are not impaired following SD (Hennecke et al., 2020[41]). SD does not impair attention, response inhibition capacity, and working memory performance in human subjects (Giacobbo et al., 2016[29]). SD also impairs objective but not self-estimated working memory performance in women, whereas both are not changed following SD in men (Rangtell et al., 2019[89]). It has also been shown that slow wave sleep restriction or RSD does not affect sleep-dependent memory consolidation (Genzel et al., 2009[27]). RSD does not impair spatial learning in the Morris water maze task in rats (Cakir et al., 2020[15]). A previous study has reported that RSD does not block re-consolidation of fear conditioning (Tian et al., 2009[107]). Also, RSD does not impair emotional verbal memory in young healthy subjects (Morgenthaler et al., 2014[73]). Some studies have reported that RSD does not impair fear memory (Silvestri, 2005[100], Silvestri and Root, 2008[101]). Importantly, SD increases the activation of the amygdala in response to emotional stimuli, due to a disconnection between the amygdala and the prefrontal cortex, leading to a potential enhancement effect on negative emotional memory (Yoo et al., 2007[123]). Furthermore, SD induces a similar hyper-responsivity of the amygdala to emotional faces (increase of negative emotional reaction) as well as reduce its functional connectivity with the ventral anterior cingulate cortex (Motomura et al., 2013[74]). It has also been shown that sleep restriction enhances the activation of the amygdala for subliminal signals of fear, indicating that SD enhances subliminal emotion processing that engages the amygdala and its connection to the superior colliculus (Motomura et al., 2014[75]). Another research has shown an increase in behavioral and neural reactivity to emotional images in RSD subjects (Rosales-Lagarde et al., 2012[93]). Furthermore, compared to young controls, sleep-deprived young adults show intact recognition rates for negative emotion memories (Vargas et al., 2019[110]). Interestingly, the left frontal, the right frontal, and the left parietal lobes are more active after TSD or early night SD, leading to higher scores for word retrieval in students (Tantawy et al., 2013[106]). A previous study has reported that SD shows conflicting results for working memory performance, indicating some studies have shown a decrease in working memory performance, and other studies have reported no effect (de Bruin et al., 2017[20]). A better performance in a verbal memory test in sleep restricted children has also been shown in a past study (Randazzo et al., 1998[88]) (Table 2(Tab. 2); References in Table 2: Azogu et al., 2015[10]; Biggs et al., 2010[12]; Cakir et al., 2020[15]; Cheng et al., 2015[19]; Giacobbo et al., 2016[29]; Gisquet-Verrier and Smith, 1989[30]; Hennecke et al., 2020[41]; Hussaini et al., 2009[42]; Mahboudi et al., 2019[64]; Morgenthaler et al., 2014[73]; Randazzo et al., 1998[88]; Rangtell et al., 2019[89]; Voderholzer et al., 2011[114]; Zhang et al., 2020[127]).
The Mechanisms Underlying the Effects of SD on Learning and Memory
Mechanisms underlying destructive effects
As mentioned, SD usually impairs learning and memory processing. SD induces a deleterious effect on proliferation of newly born neuronal cells in the hippocampus (Meerlo et al., 2009[69]), and this effect can lead to attenuated memory formation. It has been shown that SD significantly reduces neurogenesis in the hippocampus, leading to memory decline (Wadhwa et al., 2019[115]). A previous research has reported that SD induces shrinkage and loss of hippocampal neurons in mice, and impairs novel object recognition and object location memories (Wang et al., 2020[120]). Furthermore, SD disturbs hippocampal neuronal excitability in young APP/PS1 mice and impairs spatial memory performance (Tabassum et al., 2019[103]). The memory impairment effect of SD through attenuating hippocampal neurogenesis has also been reported (Guzman-Marin et al., 2005[34]; Lopez-Virgen et al., 2015[62]). 48 hours RSD reduces viable neurons in the hippocampus of mice and impairs memory performance in the Y-maze task (Olonode et al., 2019[80]). Furthermore, SD reduces neuronal cell proliferation and differentiation (Wadhwa et al., 2017[116]). SD highly affects a wide-range of hippocampal signaling pathways including transcriptional and translational processes (Vecsey et al., 2012[112]; Tudor et al., 2016[109]). Interestingly, SD dramatically affects hippocampal memory consolidation in the first few hours following training, when it overlaps with the second wave of cAMP (cyclic adenosine monophosphate) signaling, transcription, and protein synthesis critical for enhancing synaptic plasticity and memory storage (Havekes and Abel, 2017[38]). For example, 3 hours SD commencing 1 hour after training impairs the formation of spatial memory, while 3 hours after training do not alter memory consolidation (Prince et al., 2014[85]). SD decreases cAMP levels in the CA1 region of the hippocampus (Vecsey et al., 2009[111]). SD can also induce changes in second messenger pathways such as the cAMP-PKA (protein kinase A) signaling pathway (Florian et al., 2011[24]) and synaptic structure (Havekes et al., 2016[40]). It should be noted that, restoring cAMP levels in the hippocampus reverses sleep deprivation-induced memory impairments (Havekes and Abel, 2017[38]).
In addition, SD attenuates LTP (long-term potentiation) (Rezaie et al., 2020[91]). LTP is a critical mechanism in memory processes that modulates synaptic transmission especially in the hippocampus (McDermott et al., 2003[67]). SD disturbs cAMP-dependent forms of synaptic plasticity such as long-lasting forms of LTP and memory consolidation (Vecsey et al., 2009[111]; Havekes et al., 2014[39]). It has been reported that 72 hours TSD reduces LTP, attenuates synaptic plasticity, and impairs learning and memory in female rats (Rajizadeh et al., 2020[87]). RSD significantly reduces LTP induction in the hippocampus (Ishikawa et al., 2006[44]). TSD or RSD highly attenuates synaptic plasticity through decrease in PKA activity and phosphorylation of CREB (critical for long-term synaptic plasticity) in the hippocampus (Alhaider et al., 2011[3]). It has been reported that SD reduces neuronal plasticity in the hippocampus by decreasing intracellular cAMP-PKA signaling, leads to changes in CREB-mediated gene transcription, neurotrophic signaling, and glutamate receptor expression (Kreutzmann et al., 2015[56]). CREB is essential for hippocampal synaptic plasticity and LTP, and SD alters its level in the hippocampus, the striatum, and the cortex (Wang et al., 2010[118]; Abel et al., 2013[1]; Duncan et al., 2013[22]). It has been shown that CREB phosphorylation is decreased after 5 to 6 hours of TSD or RSD in the hippocampus (Vecsey et al., 2009[111]; Zhao et al., 2010[128]). SD selectively impairs PKA-dependent forms of LTP in the hippocampus (Vecsey et al., 2009[111]). A previous study has demonstrated that maternal SD attenuates LTP in the CA1 hippocampal region and reduces basal synaptic transmission (Peng et al., 2016[83]). The impairment effect of SD on LTP may be related to the disruption of cAMP signaling (Vecsey et al., 2009[111]).
SD also aggravates oxidative stress and inflammation. SD increases oxidized glutathione levels, reduced oxidized glutathione/glutathione ratio, and diminishes catalase and glutathione peroxidase activity, leading to hippocampal oxidative stress (Silva et al., 2004[99]; Alzoubi et al., 2012[7]). It has been reported that 72 hours SD significantly increases the production of ROS (reactive oxygen species) in the hippocampus (Salehpour et al., 2018[94]). Also, SD increases oxidative stress in the hippocampus via attenuating the activation of SOD (superoxide dismutase) (Zhang et al., 2013[126]). Acute SD induces oxidative stress and subsequent memory impairments (Aleisa et al., 2011[2]). A previous research has shown that SD impairs object location recognition and passive avoidance memories via increasing oxidative stress in the brain (Zhou et al., 2020[129]). It has also been revealed that 5-day TSD induces spatial memory decline in the Morris water maze task through increasing oxidative stress in the hippocampus of rats (Wang et al., 2018[119]). RSD via increasing ROS during wakefulness and induction of oxidative stress impairs memory function (Alzoubi et al., 2017[9], 2019[6]). Furthermore, SD increases pro-inflammatory cytokines in the hippocampus (Wadhwa et al., 2017[116]). SD can also impair memory performance via induction of autophagy in the hippocampus (Yang et al., 2019[122]). Other studies have shown that SD induces autophagy and apoptosis in the hippocampus (Cao et al., 2019[16], 2020[17]).
Studies have shown that SD reduces the level of BDNF (brain-derived neurotrophic factor) in the brain (Rahmani et al., 2020[86]). BDNF increases the frequency of miniature excitatory postsynaptic currents (EPSCs) in the brain (Binder and Scharfman, 2004[13]). The critical role of BDNF in the induction of LTP has also been revealed (Korte et al., 1996[54]; Patterson et al., 1996[81]). It has been shown that BDNF knockout animals show LTP impairment (Korte et al., 1995[53]). BDNF significantly modulates both sleep and memory (Mascetti et al., 2013[66]; Giacobbo et al., 2016[29]). A previous study has shown that 24 hours RSD reduces BDNF levels in the hippocampus and the prefrontal cortex (Mahboubi et al., 2019[64]). TSD downregulates BDNF expression in the hippocampus and impairs spatial memory formation in rats (Duan et al., 2016[21]). The expression of BDNF and its receptor, TrkB (tropomyosin receptor kinase) is decreased following short-term SD in the hippocampus and the amygdala of mice, leading to attenuated memory retrieval (Sharma et al., 2020[96]). Another study has shown that 7-day SD reduces the level of BDNF and TrkB in the hippocampus of mice, leading to impaired memory performance in the step-down avoidance and the Morris water maze tests (Hwang et al., 2019[43]). 24 hours SD decreases the level of BDNF in the CA1 region of the hippocampus in rats and impairs long-term memory (Zagaar et al., 2013[124]). Also, in sleep-deprived university students, BDNF plasma level is significantly lower than in normal students (Kuhn et al., 2016[58]).
Mechanisms underlying beneficial effects
The reasons beyond the beneficial effects of SD on learning and memory are ambiguous. Although most studies have shown that SD impairs learning and memory, some conflicting results on the effect of SD on learning and memory have been reported. One of the inconsistent findings in related studies is the different impact of SD on cognitive capability and expression of some neurochemicals in various regions of the brain (Mahboubi et al., 2019[64]). Interestingly, it has been reported that perioperative sleep fragmentation highly elevates hippocampal inflammation without further memory impairment, while sleep fragmentation or surgery independently induces a significant memory impairment effect (Zhang et al., 2020[125]). It has been shown that SD via increasing IL-6 (interleukin-6) improves learning and memory performance, indicating elevated IL-6 decreases tau phosphorylation through increasing FOXQ1 in the hippocampus (Zhang et al., 2020[127]). FOXQ1 is the direct target gene of miR-125b, involved in neural apoptosis and the phosphorylation of tau (Ma et al., 2017[63]). In fact, SD can indirectly improve memory performance. Importantly, the relation between IL-6 and SD is so controversial. For example, it has been shown that IL-6 level is similar between control and SD subjects (Vgontzas et al., 2002[113]). Moreover, a significant shift of the major peak of IL-6 secretion from midnight (4 a.m.) to evening (7 p.m.) has been revealed (Vgontzas et al., 2002[113]). However, a previous study has shown that sleep onset is related to an increase in serum levels of IL-6 (Redwine et al., 2000[90]). It has also been shown that different stages of sleep have a different effect on IL-6 serum level (Redwine et al., 2000[90]). In healthy sleep-deprived volunteers, the level of IL-6 in the plasma is increased (Haack et al., 2007[35]). However, it has been shown that 6-12 hours short-term SD prior to cerebral ischemia induces neuroprotective effects via decreasing inflammatory responses and glial reactions in the hippocampus of rats (Weil et al., 2009[121]; Moldovan et al., 2010[70]). It seems that the effect of SD on inflammation may significantly depend on the time window and duration of SD (Cheng et al., 2015[19]).
A previous study has shown that short-term SD increases the level of BDNF in the hippocampus of rats (Cheng et al., 2015[19]). Other studies have also shown that 12 hours short-term SD increases the expression level of hippocampal BDNF (Fujihara et al., 2003[25]; Hairston et al., 2004[37]). Furthermore, 24 hours RSD enhances the level of BDNF and TrkB in the hippocampus and the prefrontal cortex of rats exposed to intensive exercise, leading to memory improvement (Mahboubi et al., 2019[64]). In line with these results, it has been shown that RSD increases the level of BDNF in the hippocampus (Jiang and Zhu, 2015[47]). In a previous study, the sleep deprived human subjects showed higher BDNF levels and normal performance on attention, response inhibition capacity, and working memory (Giacobbo et al., 2016[29]). It has also been shown that 8 hours TSD does not change the level of BDNF in rats (Taishi et al., 2001[104]). Therefore, the inconsistent effects of SD on the level of BDNF can be involved in various effects of SD on memory performance. Sleep homeostatic and circadian mechanisms referring to the two process models of sleep regulation can be a suggested mechanism that is associated with the enhancement effect of SD on the BDNF level in the hippocampus (Kavcic et al., 2011[49]; Mahboubi et al., 2019[64]).
Note that, at least 10 genes needed for mammalian circadian clock function have been identified (Kavcic et al., 2011[49]). The positive modulators in mammals are Clock and Bmal1, while Per1-3, Cry1-2, and Dec1-2 are involved in the negative feedback loop (Shearman et al., 2000[97]; Gachon et al., 2004[26]). 40 hours acute SD affects the expression of hPer2 (a clock gene) in PBMCs (peripheral blood mononuclear cells) and leads to circadian rhythm disturbances (Kavcic et al., 2011[49]). A significant variation in hPer2 and hBmal1 expression levels under SD conditions has been reported in a previous study (Kavcic et al., 2011[49]). Importantly, BDNF-mediated signaling has an important role in the regulation of circadian rhythm (Liang et al., 2000[59]). BDNF modulates circadian pacemaker function via its localization in the SCN (Liang et al., 1998[60]). The relation between the level of BDNF and circadian gene expression has also been revealed (Moravcova et al., 2020[72]). It has been reported that BDNF is rhythmically expressed within the SCN, indicating its level is increased during the subjective night, when light shifts the phase of circadian rhythms, but is low throughout the subjective day (Liang et al., 1998[61]). BDNF plays a critical role in the time-dependent regulation of the circadian rhythm by light (Liang et al., 2000[59]). BDNF level is significantly related to obesity and neurodegeneration, and these pathologies are characterized by disrupted circadian rhythm (Genzer et al., 2016[28]). A previous finding has shown that disrupted circadian rhythm reduces the expression of BDNF and TrkB in the hippocampus of mice (Kim et al., 2019[50]). Whereas, another study has shown that clock knockdown in hippocampal neurons upregulates BDNF mRNA by 86 % (Genzer et al., 2016[28]). Importantly, it should be noted that IL-6 exhibits a circadian rhythm (Nilsonne et al., 2016[78]), and is known as a possible sleep regulatory substance (Krueger 2008[57]). As mentioned, the effect of SD on IL-6 can be a possible mechanism for the improvement effect of SD on memory function.
It has also been suggested that heightened arousal and/or SD-induced stress can have a role in the improvement effect of SD on memory (Azogu et al., 2015[10]). Acute stress can enhance associative learning in rodents (Shors, 2001[98]). Interestingly, SD can induce a stress and heighten arousal via activating the locus coeruleus noradrenergic system, leading to sensitized postsynaptic noradrenergic receptors in critical neuroplastic circuits, which in turn, facilitates arousal and augments attentional processes (Payne et al., 2002[82]). Furthermore, it has been shown that repeated periods of 4 hours SD during five days have no effect on avoidance learning, suggesting that animals can adopt to this condition via sleep rebound over the rest of the day (Borbely et al., 1984[14]; Tobler and Borbely, 1990[108]). Additionally, as previously mentioned, a previous study has reported that short-term SD stimulates hippocampal neurogenesis in rats (Cheng et al., 2015[19]). Another study has also shown this effect (Grassi Zucconi et al., 2006[31]); although more studies are needed, but this effect can lead to a better memory performance and a better mood state (Grassi et al., 2006[31]) (Table 3(Tab. 3); References in Table 3: Cheng et al., 2015[19]; Duan et al., 2016[21]; Fujihara et al., 2003[25]; Giacobbo et al., 2016[29]; Grassi Zucconi et al., 2006[31]; Guzman-Marin et al., 2005[34]; Hairston et al., 2004[37], 2005[36]; Hwang et al., 2019[43]; Junek et al., 2010[48]; Kavcic et al., 2011[49]; Kuhn et al., 2016[58]; Mahboubi et al., 2019;[64] Olonode et al., 2019[80]; Sharma et al., 2020[96]; Taishi et al., 2001[104]; Wadhwa et al., 2019[115]; Wang et al., 2020[120]; Zagaar et al., 2013[124]).
Concluding Remarks and Future Perspective
Eventually, an important question remains about the role of SD in memory processing: Is SD destructive or beneficial for memory function? As mentioned throughout the manuscript, most of the studies have shown the impairment effects of SD (TSD and RSD) on learning and memory performance. However, some studies have shown the opposite results, indicating that SD improves learning and memory. This is a critical dilemma. We should find the biological and cellular reasons beyond these inconsistent results. We mentioned to several mechanisms underlying the impairment effect of SD on memory processing. We also mentioned to some mechanisms underlying the improvement effect of SD on memory processing. Thus, we should focus on similarities between these conflicting studies. These similarities are as follows: 1) Neurogenesis: as mentioned, SD may induce dualistic effects on neurogenesis in the hippocampus. SD has shown both improvement and impairment effects on neurogenesis in the hippocampus. On the other hand, neurogenesis induces a region-specific response to SD. Importantly, as Table 3(Tab. 3) shows, it seems that short-term SD stimulates neurogenesis, while long-term SD induces an impairment effect, concluding that the duration of SD plays an important role in cell proliferation changes following SD. Thus, it seems that the duration of SD may have a critical role in the modulation of neurogenesis. However, more detailed studies are needed to prove this point. 2) BDNF: as mentioned, BDNF has a critical role in the effects of SD on memory. On the other hand, SD can alter the expression level of BDNF and its receptor, TrkB. Many studies have shown that SD reduces the expression of BDNF, while some studies have shown the opposite result. The upregulation of BDNF can trigger the neurogenesis in the hippocampus, leading to a better memory performance. Importantly, disrupted circadian rhythm following SD can affect the expression level of BDNF. Thus, the effect of SD or disrupted circadian rhythm on the expression level of BDNF and subsequent neurogenesis may have an important role in the impact of SD on memory processing. 3) Circadian genes: as mentioned, BDNF-mediated signaling plays a crucial role in circadian regulation. Also, SD significantly affects the normal rhythm of the circadian cycle. Disrupted circadian rhythm and any disturbances in the expression of circadian genes can induce both increment or decrement effect on the BDNF level, leading to inconsistent effects on memory. 4) IL-6: as mentioned, the relation between IL-6 and SD is so controversial. Inflammation plays a critical role in response to SD. During SD, the level of IL-6 shows a variation and its change depends on the time. SD also induces inconsistent effects on the level of IL-6, leading to various effects on memory function. Importantly, the effect of SD on inflammation significantly depends on the time window and duration of SD. Also, the role of IL-6 in modulating circadian rhythm is so important and should be considered in future studies. 5) Stress: as mentioned, heightened arousal and/or SD-induced stress can have a role in the improvement effect of SD on memory. However, this stress can also induce an impairment effect on memory performance, because stress by itself, can induce an adverse effect on neurogenesis. Also, adaptation of animals to SD condition may alter their memory performance and neutralize the adverse effect of stress on memory function. Thus, the role of SD-induced stress is so important and can dramatically affect learning and memory performance.
Conflict of interest
The authors declare that they have no conflict of interest.
Funding information
There is no providing financial support to this project.
References
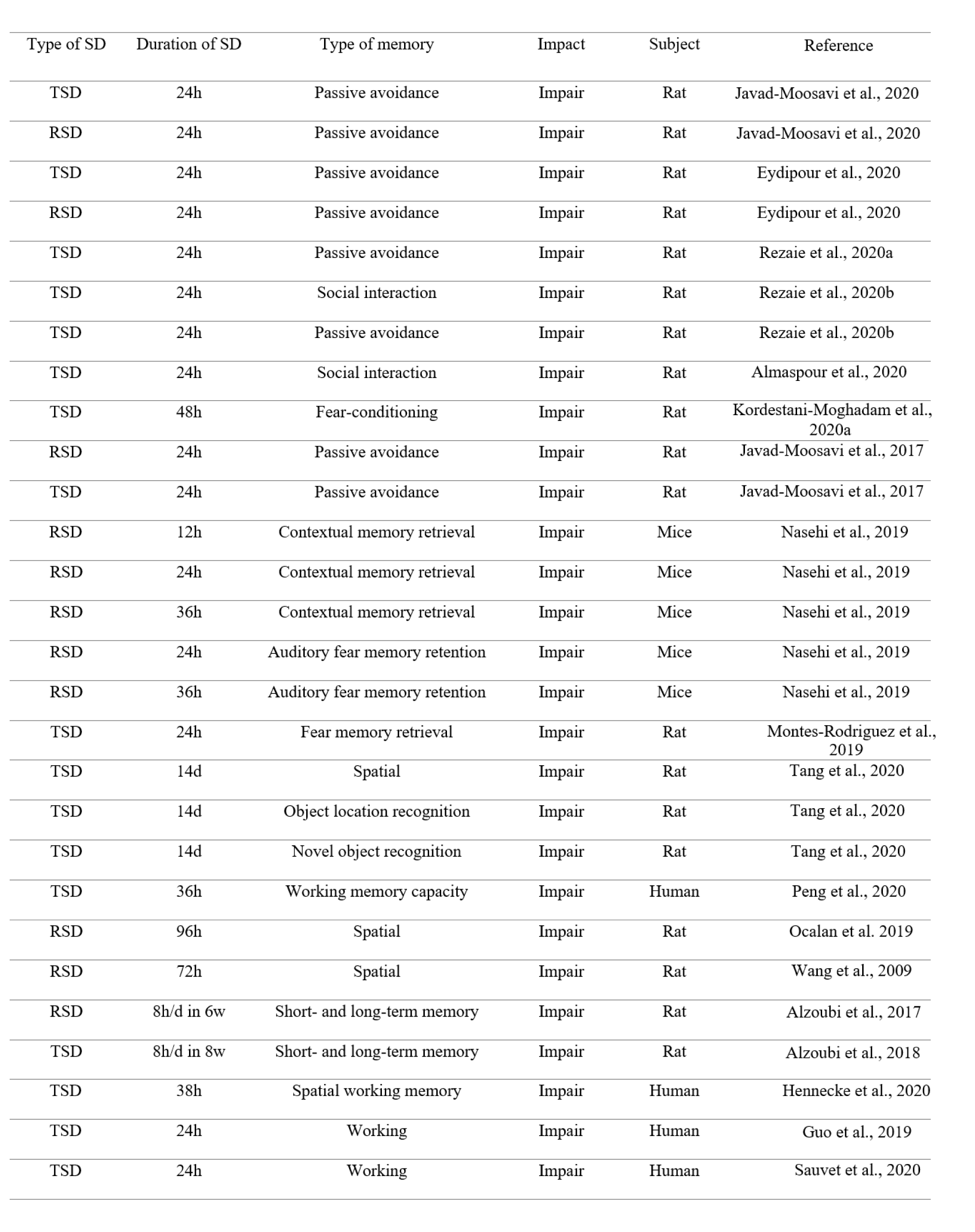
Table 1: The impairment effect of TSD or RSD on different types of memory (h = hour, d = day, w = week, SD = sleep deprivation, TSD = total sleep deprivation, RSD = REM sleep deprivation)
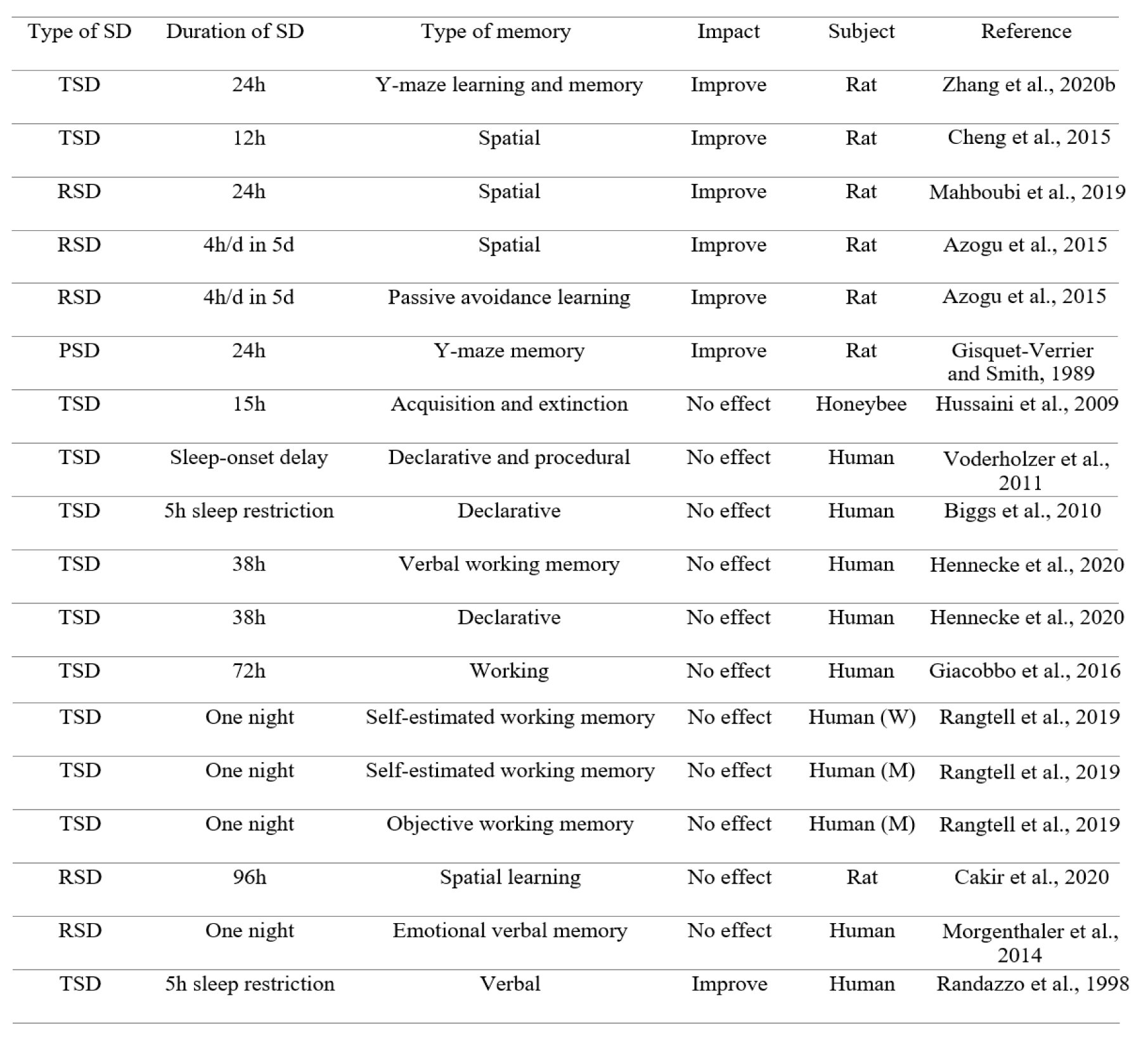
Table 2: The improvement (or no impairment) effect of TSD or RSD on different types of memory (h = hour, d = day, SD = sleep deprivation, TSD = total sleep deprivation, RSD = REM sleep deprivation, PSD = paradoxical sleep deprivation, M = Men, W = Women)
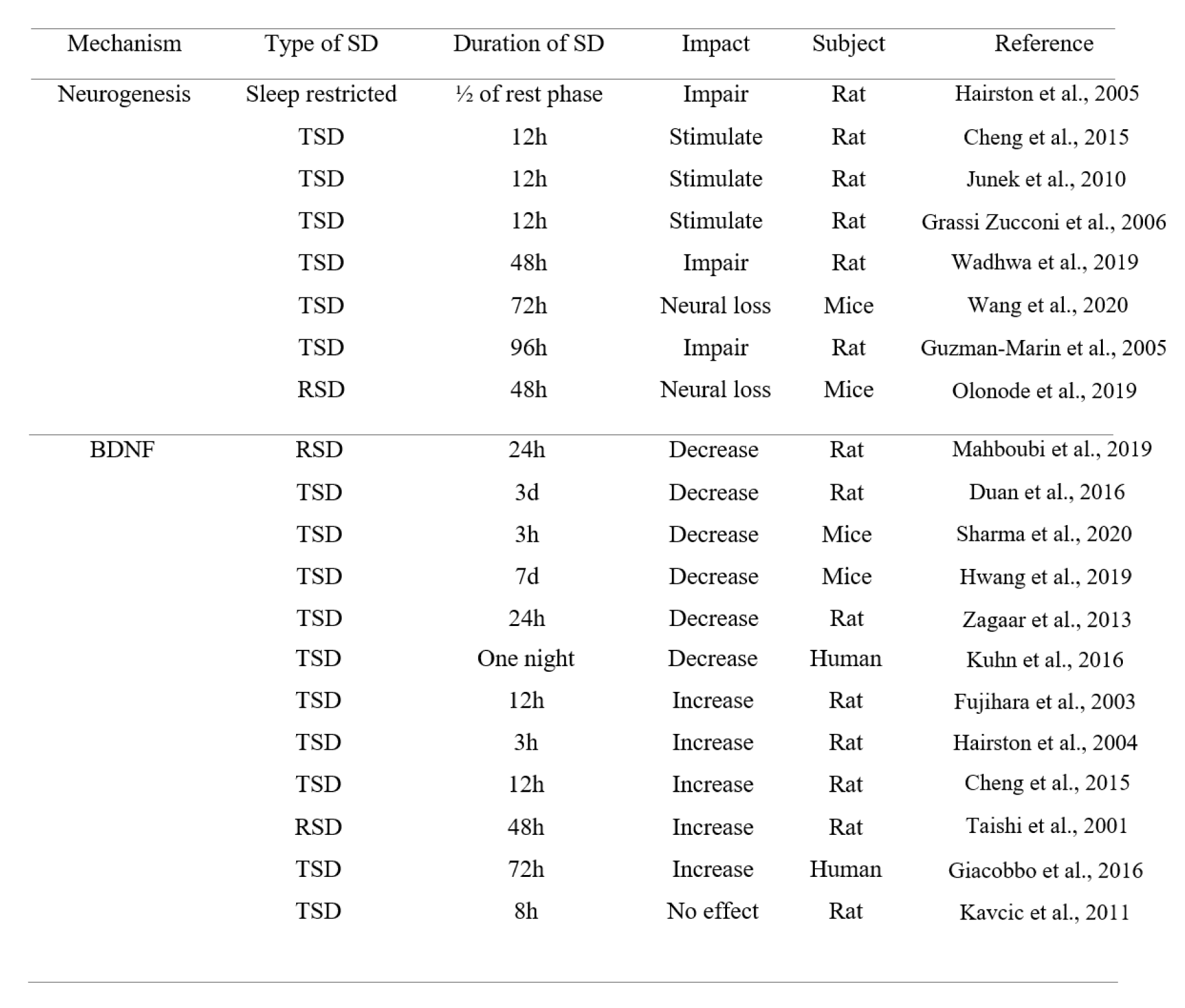
Table 3: The inconsistent effects of TSD or RSD on neurogenesis and BDNF level (h = hour, d = day, SD = sleep deprivation, TSD = total sleep deprivation, RSD = REM sleep deprivation)
[*] Corresponding Author:
Salar Vaseghi, Cognitive and Neuroscience Research Center (CNRC), Amir-Almomenin Hospital, Tehran Medical Sciences, Islamic Azad University, Tehran, Iran, P.O. Box: 13145-784; Tel: +9821-99881118-20, Fax: +9821-99881117, eMail: salarv67@yahoo.com