Letter to the editor
A recent overview on ginsenosides as microRNA modulators in the treatment of human diseases
Tae Kyung Hyun1
1Department of Industrial Plant Science and Technology, College of Agricultural, Life and Environmental Sciences, Chungbuk National University, Cheongju 28644, Republic of Korea
EXCLI J 2021;20:Doc1453
Dear Editor,
MicroRNAs (miRNAs) are short (20-22 nucleotides) and highly conserved noncoding transcripts that play a crucial role in the regulation of gene expression, guiding the RNA-induced silencing complex to target mRNAs (Treiber et al., 2019[26]). Under normal physiological conditions, miRNAs are involved in feedback and feedforward loops, which have widespread functions in diverse biological processes, including cell proliferation, differentiation, and apoptosis (Tsang et al., 2007[27]; Reddy, 2015[23]). Since the human disease-related miR15 and miR16, located at chromosome 13q14, were first characterized in chronic lymphocytic leukemia (Calin et al., 2002[4]), much attention has been directed towards the function of miRNAs in a number of disorders such as cancer, viral infections, diabetes, immune-related diseases, and neurodegenerative disorders (Condrat et al., 2020[12]). In addition, accumulating evidence suggests that miRNA-mediated control of gene expression is important for the treatment of various diseases (Ali Syeda et al., 2020[1]; Condrat et al., 2020[12]; Zhang et al., 2020[37]; Wang et al., 2021[31]).
Ginsenosides are a class of steroid glycosides and triterpene saponins that account for the medical effects of ginseng (Panax ginseng). Among more than a hundred ginsenosides in ginseng, the most abundant ginsenosides are Rb1, Rb2, Rc, Rd, Re, and Rg1, all of which belong to the protopanaxadiol or protopanaxatriol saponins (Chen et al., 2019[5]). A growing body of evidence indicates that ginsenosides act as antioxidant, antimicrobial, anti-inflammatory, anti-cancer, anti-diabetic, and anti-aging agents, although each ginsenoside exhibits a different pharmacological action (Bai et al., 2018[2]; Zheng et al., 2018[39]; Wang and Roh, 2020[29]). The molecular targets of these effects contain various signaling pathways, including the Ras/Raf/MEK/ERK, PI3K/Akt, NF-κB, and PPARγ/HO-1 signaling pathways (Bai et al., 2018[2]; Zheng et al., 2018[38]). In addition, increasing focus on ginsenosides as miRNA modulators continues to contribute to advances in clinical trials.
In this letter, we present a review of recent clinical findings on the miRNA-mediated pharmacological role of ginsenosides (Table 1(Tab. 1); References in Table 1: Cai et al., 2019[3]; Cheng and Xing, 2019[10]; Chen et al., 2018[6][7], 2019[9], 2021[8]; Chu et al., 2019[11]; Gao and Zheng, 2018[13]; Jia et al., 2019[14]; Jiang et al., 2021[15]; Kim et al., 2017[17], 2021[16]; Lee et al., 2020[18]; Li et al., 2019[19]; Liang et al., 2019[20]; Liu et al., 2020[21]; Paik et al., 2019[22]; Shi et al., 2018[25], 2019[24]; Wang et al., 2018[30], 2021[28]; Wilkes et al., 2021[32]; Xue et al., 2018[33]; Yang et al., 2019[34]; Yi et al., 2019[35]; Yu et al., 2018[36]; Zheng et al., 2018[38]; Zhou et al., 2018[40]). We believe that this letter provides a solid foundation for further evaluation of ginsenosides as miRNA modulators in the prevention and treatment of a number of chronic diseases in humans.
Conflict of interest
The authors declare no conflict of interest.
References
1.
Ali Syeda Z, Langden SSS, Munkhzul C, Lee M, Song SJ. Regulatory mechanism of microRNA expression in cancer. Int J Mol Sci.2020;21:1723. 2.
Bai L, Gao J, Wei F, Zhao J, Wang D, Wei J. Therapeutic potential of ginsenosides as an adjuvant treatment for diabetes. Front Pharmacol. 2018;9:423. 3.
Cai HA, Huang L, Zheng LJ, Fu K, Wang J, Hu FD, et al. Ginsenoside (Rg-1) promoted the wound closure of diabetic foot ulcer through iNOS elevation via miR-23a/IRF-1 axis. Life Sci. 2019;233:116525. 4.
Calin GA, Dumitru CD, Shimizu M, Bichi R, Zupo S, Noch E, et al. Frequent deletions and down-regulation of micro-RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc Natl Acad Sci U S A. 2002;99:15524-9.5.
Chen W, Balan P, Popovich DG. Analysis of ginsenoside content (Panax ginseng) from different regions. Molecules. 2019;24:3491.6.
Chen W, Chu S, Li H, Qiu Y. MicroRNA-146a-5p enhances ginsenoside Rh2-induced anti-proliferation and the apoptosis of the human liver cancer cell line HepG2. Oncol Lett. 2018;16:5367-74.7.
Chen Y, Shang H, Zhang S, Zhang X. Ginsenoside Rh2 inhibits proliferation and migration of medulloblastoma Daoy by down-regulation of microRNA-31. J Cell Biochem. 2018;119:6527-34.8.
Chen Y, Wang S, Yang S, Li R, Yang Y, Chen Y, et al. Inhibitory role of ginsenoside Rb2 in endothelial senescence and inflammation mediated by microRNA‑216a. Mol Med Rep. 2021;23:415. 9.
Chen Y, Zhang Y, Song W, Zhang Y, Dong X, Tan M. Ginsenoside Rh2 inhibits migration of lung cancer cells under hypoxia via miR-491. Anticancer Agents Med Chem. 2019;19:1633-41.10.
Cheng Z, Xing D. Ginsenoside Rg3 inhibits growth and epithelial-mesenchymal transition of human oral squamous carcinoma cells by down-regulating miR-221. Eur J Pharmacol. 2019;853:353-63. 11.
Chu SF, Zhang Z, Zhou X, He WB, Chen C, Luo P, et al. Ginsenoside Rg1 protects against ischemic/reperfusion-induced neuronal injury through miR-144/Nrf2/ ARE pathway. Acta Pharmacol Sin. 2019;40:13-25. 12.
Condrat CE, Thompson DC, Barbu MG, Bugnar OL, Boboc A, Cretoiu D, et al. miRNAs as biomarkers in disease: Latest findings regarding their role in diagnosis and prognosis. Cells. 2020;9:276. 13.
Gao Q, Zheng J. Ginsenoside Rh2 inhibits prostate cancer cell growth through suppression of microRNA-4295 that activates CDKN1A. Cell Prolif. 2018;51:e12438.14.
Jia F, Mou L, Ge H. Protective effects of ginsenoside Rb1 on H2O2-induced oxidative injury in human endothelial cell line (EA.hy926) via miR-210. Int J Immunopathol Pharmacol. 2019;33:2058738419866021.15.
Jiang M, Zhu Y, Yu H. Ginsenoside 20(S)-Rg3 suppresses cell viability in esophageal squamous cell carcinoma via modulating miR-324-5p-targeted PSME3. Hum Exp Toxicol. 2021;epub ahed of print. 16.
Kim H, Ji HW, Kim HW, Yun SH, Park JE, Kim SJ. Ginsenoside Rg3 prevents oncogenic long noncoding RNA ATXN8OS from inhibiting tumor-suppressive microRNA-424-5p in breast cancer cells. Biomolecules. 2021;11:118. 17.
Kim MK, Lee SK, Park JH, Lee JH, Yun BH, Park JH, et al. Ginsenoside Rg3 decreases fibrotic and invasive nature of endometriosis by modulating miRNA-27b: In vitro and in vivo studies. Sci Rep. 2017;7:17670. 18.
Lee A, Yun E, Chang W, Kim J. Ginsenoside Rg3 protects against iE-DAP-induced endothelial-to-mesenchymal transition by regulating the miR-139-5p-NF-κB axis. J Ginseng Res. 2020;44:300-7. 19.
Li M, Zhang D, Cheng J, Liang J, Yu F. Ginsenoside Rh2 inhibits proliferation but promotes apoptosis and autophagy by down-regulating microRNA-638 in human retinoblastoma cells. Exp Mol Pathol. 2019;108:17-23. 20.
Liang H, Zhang S, Li Z. Ginsenoside Rg3 protects mouse leydig cells against triptolide by downregulation of miR-26a. Drug Des Devel Ther. 2019;13:2057-66. 21.
Liu GM, Lu TC, Sun ML, Jia WY, Ji X, Luo YG. Ginsenoside Rd inhibits glioblastoma cell proliferation by up-regulating the expression of miR-144-5p. Biol Pharm Bull. 2020;43:1534-41.22.
Paik S, Choe JH, Choi GE, Kim JE, Kim JM, Song GY, et al. Rg6, a rare ginsenoside, inhibits systemic inflammation through the induction of interleukin-10 and microRNA-146a. Sci Rep. 2019;9:4342. 23.
Reddy KB. MicroRNA (miRNA) in cancer. Cancer Cell Int. 2015;15:38.24.
Shi Q, Chen X, Sun G, Wang L, Cui L. Ginsenoside Rg1 protects human retinal pigment epithelial ARPE-19 cells from toxicity of high glucose by up-regulation of miR-26a. Life Sci. 2019;221:152-8. 25.
Shi R, Zhang S, Cheng G, Yang X, Zhao N, Chen C. Ginsenoside Rg1 and Acori graminei rhizoma attenuates neuron cell apoptosis by promoting the expression of miR-873-5p in Alzheimer's disease. Neurochem Res. 2018;43:1529-38. 26.
Treiber T, Treiber N, Meister G. Regulation of microRNA biogenesis and its crosstalk with other cellular pathways. Nat Rev Mol Cell Biol. 2019;20:5-20. 27.
Tsang J, Zhu J, van Oudenaarden A. MicroRNA-mediated feedback and feedforward loops are recurrent network motifs in mammals. Mol Cell. 2007;26:753-67.28.
Wang D, Zhao S, Pan J, Wang Z, Li Y, Xu X, et al. Ginsenoside Rb1 attenuates microglia activation to improve spinal cord injury via microRNA-130b-5p/TLR4/NF-κB axis. J Cell Physiol. 2021;236:2144-55.29.
Wang F, Roh YS. Mitochondrial connection to ginsenosides. Arch Pharm Res. 2020;43:1031-45. 30.
Wang J, Li D, Hou J, Lei H. Protective effects of geniposide and ginsenoside Rg1 combination treatment on rats following cerebral ischemia are mediated via microglial microRNA‑155‑5p inhibition. Mol Med Rep. 2018;17:3186-93.31.
Wang S, Shen L, Luo H. Identification and validation of key miRNAs and a microRNA-mRNA regulatory network associated with ulcerative colitis. DNA Cell Biol. 2021;40:147-56.32.
Wilkes Mc, Jung K, Lee BE, Saxena M, Sathianathen RS, Mercado JD, et al. The active component of ginseng, ginsenoside Rb1, improves erythropoiesis in models of Diamond Blackfan Anemia by targeting Nemo-like Kinase. J Biol Chem. 2021;297(3):100988.33.
Xue LP, Fu XL, Hu M, Zhang LW, Li YD, Peng YL, et al. Rg1 inhibits high glucose-induced mesenchymal activation and fibrosis via regulating miR-2113/RP11-982M15.8/Zeb1 pathway. Biochem Biophys Res Commun. 2018;501:827-32. 34.
Yang C, Li B, Liu Y, Xing Y. Ginsenoside Rb1 protects cardiomyocytes from oxygen-glucose deprivation injuries by targeting microRNA-21. Exp Ther Med. 2019;17:3709-16. 35.
Yi G, Liu L, Lv C, Wei Y, Yan T. Ginsenoside Rg1 defenses PC-12???cells against hydrogen peroxide-caused damage via up-regulation of miR-216a-5p. Life Sci. 2019;236:116948. 36.
Yu H, Fan C, Yang L, Yu S, Song Q, Wang P, et al. Ginsenoside Rg1 prevents chronic stress-induced depression-like behaviors and neuronal structural plasticity in rats. Cell Physiol Biochem. 2018;48:2470-82. 37.
Zhang B, Tian L, Xie J, Chen G, Wang F. Targeting miRNAs by natural products: A new way for cancer therapy. Biomed Pharmacother. 2020;130:110546.38.
Zheng HZ, Fu XK, Shang JL, Lu RX, Ou YF, Chen CL. Ginsenoside Rg1 protects rat bone marrow mesenchymal stem cells against ischemia induced apoptosis through miR-494-3p and ROCK-1. Eur J Pharmacol. 2018;822:154-67.39.
Zheng M, Xin Y, Li Y, Xu F, Xi X, Guo H, et al. Ginsenosides: A potential neuroprotective agent. Biomed Res Int. 2018;2018:8174345.40.
Zhou Y, Zheng X, Lu J, Chen W, Li X, Zhao L. Ginsenoside 20(S)-Rg3 inhibits the warburg effect via modulating DNMT3A/ MiR-532-3p/HK2 pathway in ovarian cancer cells. Cell Physiol Biochem. 2018;45:2548-59.
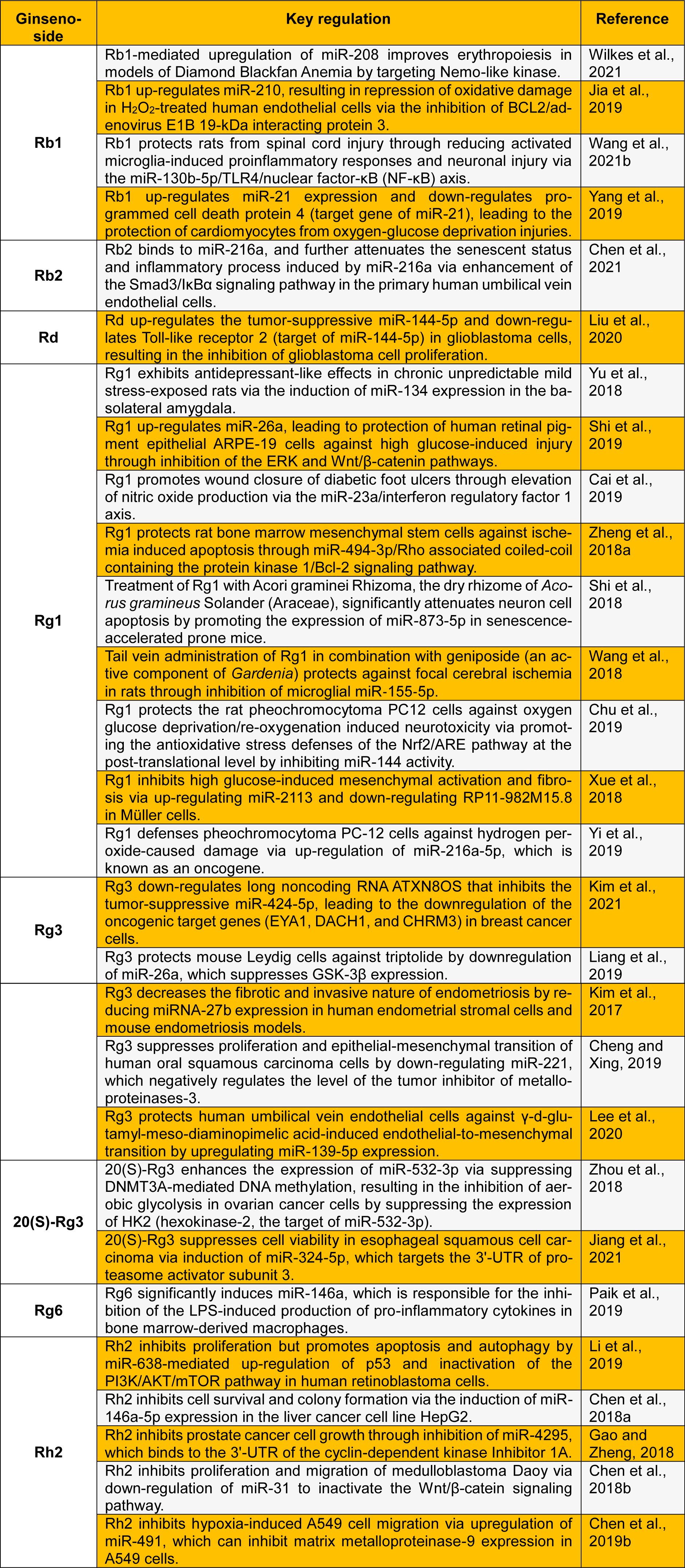
Table 1: Recent studies on the modulation of microRNAs by ginsenosides as potential therapeutics
