Letter to the editor
Current update on the protective effect of naringin in inflammatory lung diseases
Gaurav Gupta1,2, Waleed Hassan Almalki3, Imran Kazmi4, Neeraj Kumar Fuloria5, Shivkanya Fuloria5, Vetriselvan Subramaniyan6, Mahendran Sekar7, Sachin Kumar Singh8,9, Dinesh Kumar Chellappan10, Kamal Dua9,11
1School of Pharmacy, Suresh Gyan Vihar University, Jagatpura 302017, Mahal Road, Jaipur, India
2Department of Pharmacology, Saveetha Dental College, Saveetha Institute of Medical and Technical Sciences, Saveetha University, Chennai, India
3Department of Pharmacology, College of Pharmacy, Umm Al-Qura University, Makkah, Saudi Arabia
4Department of Biochemistry, Faculty of Science, King Abdulaziz University, Jeddah, Saudi Arabia
5Faculty of Pharmacy, AIMST University, Bedong 08100, Kedah, Malaysia
6Faculty of Medicine, Bioscience and Nursing, MAHSA University, Jalan SP 2, Bandar Saujana Putra, 42610 Jenjarom Selangor, Malaysia
7Department of Pharmaceutical Chemistry, Faculty of Pharmacy and Health Sciences, Royal College of Medicine Perak, Universiti Kuala Lumpur, Ipoh 30450, Perak, Malaysia
8School of Pharmaceutical Sciences, Lovely Professional University, Phagwara, Punjab, 144411, India
9Faculty of Health, Australian Research Center in Complementary and Integrative Medicine, University of Technology Sydney, Ultimo, NSW, 2007, Australia
10Department of Life Sciences, School of Pharmacy, International Medical University, Kuala Lumpur, 57000, Malaysia
11Discipline of Pharmacy, Graduate School of Health, University of Technology Sydney, NSW 2007, Australia
EXCLI J 2022;21:Doc573
Understanding the role of inflammation in developing respiratory illnesses such as COPD, asthma, and lung cancer, is critical. Natural cures are regaining favor as effective treatments for various ailments (Heidary Moghaddam et al., 2020[14]). A flavanone glycoside named naringin (NAR) is found in aromatic Chinese herbal treatments and citrus fruits. Even though several biological and pharmacological properties of NAR have been found via study, only a few systematic reviews have been published (Wadhwa et al., 2021[29]). However, there is a scarcity of studies focusing on NAR's therapeutic potential in respiratory system inflammation. NAR's alleged anti-inflammatory properties influence many pro-inflammatory cytokines, including the NF-κB, ERK1/2, and p38 MAPK pathways in the pathophysiological processes associated with chronic respiratory disorders (Chen et al., 2016[8]). This review will be very useful to researchers in this field. It will take them in a new direction in their hunt for innovative medications to treat respiratory illnesses (Table 1(Tab. 1); References in Table 1: Ahmad et al., 2015[1]; Akintunde et al., 2020[2]; Ali et al., 2017[3]; Bear and Teel, 2000[4]; Cerkezkayabekir et al., 2017[5]; Chang et al., 2017[6]; Chen et al., 2013[9], 2014[10], 2018[7]; Clementi et al., 2021[11]; Fouad et al., 2016[12]; Guihua et al., 2016[13]; Hsiao et al., 2007[15]; Jiao et al., 2015[16]; Kim et al., 2018[17]; Liu et al., 2011[20], 2012[19], 2018[18]; Luo et al., 2012[21]; Nie et al., 2012[22]; Schwarz et al., 2005[23]; Seyedrezazadeh et al., 2015[24]; Shi et al., 2014[27], 2019[25], 2021[26]; Turgut et al., 2016[28]; Wang et al., 2016[30]; Wu et al., 2021[31]; Yao et al., 2021[32]; Zhang et al., 2013[33]).
Conflict of interest
The authors declare no conflict of interest.
References
1.
Ahmad SF, Attia SM, Bakheet SA, Zoheir KM, Ansari MA, Korashy HM, et al. Naringin attenuates the development of carrageenan-induced acute lung inflammation through inhibition of NF-κb, STAT3 and pro-inflammatory mediators and enhancement of IκBα and anti-inflammatory cytokines. Inflammation. 2015;38:846-57. doi: 10.1007/s10753-014-9994-y2.
Akintunde JK, Abioye JB, Ebinama ON. Potential protective effects of naringin on oculo-pulmonary injury induced by PM10 (wood smoke) exposure by modulation of oxidative damage and acetylcholine esterase activity in a rat model. Curr Ther Res Clin Exp. 2020;92:100586. doi: 10.1016/j.curtheres.2020.1005863.
Ali R, Shahid A, Ali N, Hasan SK, Majed F, Sultana S. Amelioration of benzo[a]pyrene-induced oxidative stress and pulmonary toxicity by Naringenin in Wistar rats: A plausible role of COX-2 and NF-κB. Hum Exp Toxicol. 2017;36:349-64. doi: 10.1177/09603271166500094.
Bear WL, Teel RW. Effects of citrus phytochemicals on liver and lung cytochrome P450 activity and on the in vitro metabolism of the tobacco-specific nitrosamine NNK. Anticancer Res. 2000;20:3323-95.
Cerkezkayabekir A, Sanal F, Bakar E, Ulucam E, Inan M. Naringin protects viscera from ischemia/reperfusion injury by regulating the nitric oxide level in a rat model. Biotech Histochem. 2017;92:252-63. doi: 10.1080/10520295.2017.13054996.
Chang HL, Chang YM, Lai SC, Chen KM, Wang KC, Chiu TT, et al. Naringenin inhibits migration of lung cancer cells via the inhibition of matrix metalloproteinases-2 and -9. Exp Ther Med. 2017;13:739-44. doi: 10.3892/etm.2016.39947.
Chen M, Peng W, Hu S, Deng J. miR-126/VCAM-1 regulation by naringin suppresses cell growth of human non-small cell lung cancer. Oncol Lett. 2018;16:4754-60. doi: 10.3892/ol.2018.92048.
Chen R, Qi QL, Wang MT, Li QY. Therapeutic potential of naringin: an overview. Pharm Biol. 2016;54:3203-10. doi: 10.1080/13880209.2016.12161319.
Chen Y, Nie YC, Luo YL, Lin F, Zheng YF, Cheng GH, et al. Protective effects of naringin against paraquat-induced acute lung injury and pulmonary fibrosis in mice. Food Chem Toxicol. 2013;58:133-40. doi: 10.1016/j.fct.2013.04.02410.
Chen Y, Wu H, Nie YC, Li PB, Shen JG, Su WW. Mucoactive effects of naringin in lipopolysaccharide-induced acute lung injury mice and beagle dogs. Environ Toxicol Pharmacol. 2014;38:279-87. doi: 10.1016/j.etap.2014.04.03011.
Clementi N, Scagnolari C, D'Amore A, Palombi F, Criscuolo E, Frasca F, et al. Naringenin is a powerful inhibitor of SARS-CoV-2 infection in vitro. Pharmacol Res. 2021;163:105255-. doi: 10.1016/j.phrs.2020.10525512.
Fouad AA, Albuali WH, Jresat I. Protective effect of naringenin against lipopolysaccharide-induced acute lung injury in rats. Pharmacology. 2016;97:224-32. doi: 10.1159/00044426213.
Guihua X, Shuyin L, Jinliang G, Wang S. Naringin protects ovalbumin-induced airway inflammation in a mouse model of asthma. Inflammation. 2016;39:891-9. doi: 10.1007/s10753-016-0321-714.
Heidary Moghaddam R, Samimi Z, Moradi SZ, Little PJ, Xu S, Farzaei MH. Naringenin and naringin in cardiovascular disease prevention: A preclinical review. Eur J Pharmacol. 2020;887:173535. doi: 10.1016/j.ejphar.2020.17353515.
Hsiao YC, Kuo WH, Chen PN, Chang HR, Lin TH, Yang WE, et al. Flavanone and 2'-OH flavanone inhibit metastasis of lung cancer cells via down-regulation of proteinases activities and MAPK pathway. Chem Biol Interct. 2007;167:193-206. doi: 10.1016/j.cbi.2007.02.01216.
Jiao HY, Su WW, Li PB, Liao Y, Zhou Q, Zhu N, et al. Therapeutic effects of naringin in a guinea pig model of ovalbumin-induced cough-variant asthma. Pulm Pharmacol Ther. 2015;33:59-65. doi: 10.1016/j.pupt.2015.07.00217.
Kim JK, Park JH, Ku HJ, Kim SH, Lim YJ, Park JW, et al. Naringin protects acrolein-induced pulmonary injuries through modulating apoptotic signaling and inflammation signaling pathways in mice. J Nutr Biochem. 2018;59:10-6. doi: 10.1016/j.jnutbio.2018.05.01218.
Liu J, Yao J, Zhang J. Naringenin attenuates inflammation in chronic obstructive pulmonary disease in cigarette smoke induced mouse model and involves suppression of NF-κB. J Microbiol Biotechnol. 2018;epub ahead of print. doi: 10.4014/jmb.1810.1006119.
Liu Y, Su WW, Wang S, Li PB. Naringin inhibits chemokine production in an LPS-induced RAW 264.7 macrophage cell line. Mol Med Rep. 2012;6:1343-50. doi: 10.3892/mmr.2012.107220.
Liu Y, Wu H, Nie YC, Chen JL, Su WW, Li PB. Naringin attenuates acute lung injury in LPS-treated mice by inhibiting NF-κB pathway. Int Immunopharmacol. 2011;11:1606-12. doi: 10.1016/j.intimp.2011.05.02221.
Luo YL, Zhang CC, Li PB, Nie YC, Wu H, Shen JG, et al. Naringin attenuates enhanced cough, airway hyperresponsiveness and airway inflammation in a guinea pig model of chronic bronchitis induced by cigarette smoke. Int Immunopharmacol. 2012;13:301-7. doi: 10.1016/j.intimp.2012.04.01922.
Nie YC, Wu H, Li PB, Luo YL, Long K, Xie LM, et al. Anti-inflammatory effects of naringin in chronic pulmonary neutrophilic inflammation in cigarette smoke-exposed rats. J Med Food. 2012;15:894-900. doi: 10.1089/jmf.2012.225123.
Schwarz D, Kisselev P, Roots I. CYP1A1 genotype-selective inhibition of benzo[a]pyrene activation by quercetin. Eur J Cancer. 2005;41:151-8. doi: 10.1016/j.ejca.2004.08.01124.
Seyedrezazadeh E, Kolahian S, Shahbazfar AA, Ansarin K, Pour Moghaddam M, Sakhinia M, et al. Effects of the flavanone combination hesperetin-naringenin, and orange and grapefruit juices, on airway inflammation and remodeling in a murine asthma model. Phytother Res. 2015;29:591-8. doi: 10.1002/ptr.529225.
Shi R, Su WW, Zhu ZT, Guan MY, Cheng KL, Fan WY, et al. Regulation effects of naringin on diesel particulate matter-induced abnormal airway surface liquid secretion. Phytomedicine. 2019;63:153004. doi: 10.1016/j.phymed.2019.15300426.
Shi X, Luo X, Chen T, Guo W, Liang C, Tang S, et al. Naringenin inhibits migration, invasion, induces apoptosis in human lung cancer cells and arrests tumour progression in vitro. J Cell Mol Med. 2021;25:2563-71. doi: 10.1111/jcmm.1622627.
Shi Y, Tan Y, Mao S, Gu W. Naringenin inhibits allergen‑induced airway remodeling in a murine model of asthma. Mol Med Rep. 2014;9:1204-8. doi: 10.3892/mmr.2014.194028.
Turgut NH, Kara H, Elagoz S, Deveci K, Gungor H, Arslanbas E. The protective effect of naringin against bleomycin-induced pulmonary fibrosis in Wistar rats. Pulm Med. 2016;2016:7601393. doi: 10.1155/2016/760139329.
Wadhwa R, Paudel KR, Chin LH, Hon CM, Madheswaran T, Gupta G, et al. Anti-inflammatory and anti-cancer activities of Naringenin-loaded liquid crystalline nanoparticles in vitro. J Food Biochem. 2021;45(1):e13572. doi: 10.1111/jfbc.1357230.
Wang Y, Lu Y, Luo M, Shi X, Pan Y, Zeng H, et al. Evaluation of pharmacological relaxation effect of the natural product naringin on in vitro cultured airway smooth muscle cells and in vivo ovalbumin-induced asthma Balb/c mice. Biomed Rep. 2016;5:715-22. doi: 10.3892/br.2016.79731.
Wu Y, Cai C, Xiang Y, Zhao H, Lv L, Zeng C. Naringin ameliorates monocrotaline-induced pulmonary arterial hypertension through endothelial-to-mesenchymal transition inhibition. Front Pharmacol. 2021;12:696135. doi: 10.3389/fphar.2021.69613532.
Yao W, Zhang X, Xu F, Cao C, Liu T, Xue Y. The therapeutic effects of naringenin on bronchial pneumonia in children. Pharmacol Res Perspect. 2021;9(4):e00825. doi: 10.1002/prp2.82533.
Zhang Y, Wang JF, Dong J, Wei JY, Wang YN, Dai XH, et al. Inhibition of α-toxin production by subinhibitory concentrations of naringenin controls Staphylococcus aureus pneumonia. Fitoterapia. 2013;86:92-9. doi: 10.1016/j.fitote.2013.02.001
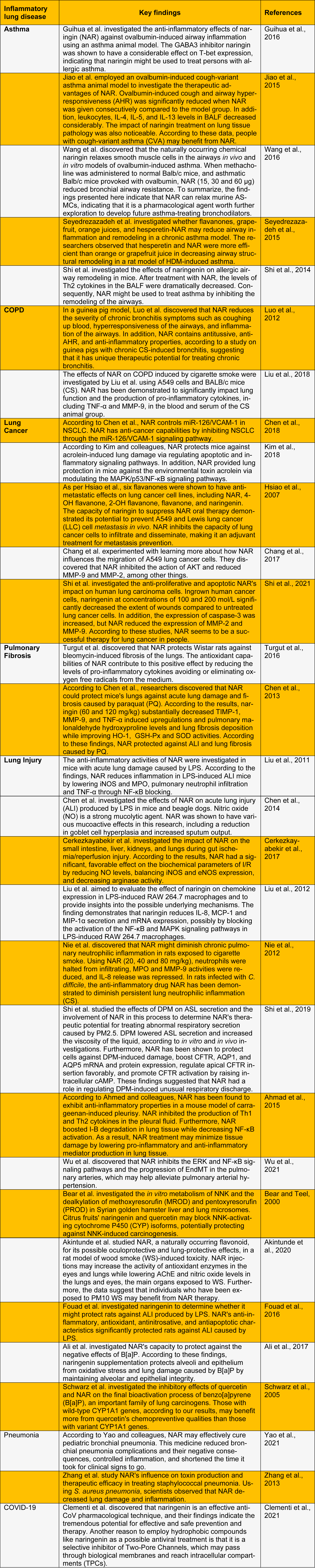
Table 1: An update on the protective effect of naringin in various inflammatory lung diseases
