Letter to the editor
Current update on anticancer effects of icariin: A journey of the last ten years
Mukta Gupta1, Yachana Mishra2, Vijay Mishra1, Murtaza M. Tambuwala3
1School of Pharmaceutical Sciences, Lovely Professional University, Phagwara (Punjab)-144411, India
2Department of Zoology, Shri Shakti Degree College, Sankhahari, Ghatampur,Kanpur Nagar (UP)-209206, India
3School of Pharmacy & Pharmaceutical Sciences, Ulster University, Coleraine, County Londonderry, BT52 1SA, Northern Ireland, United Kingdom
EXCLI J 2022;21:Doc680
Icariin (C33H40O15), a prenylated flavonoid (Figure 1(Fig. 1)), is mainly found in Chinese medicinal herbs of the family Epimedium. It is reported for diverse pharmacological activities in different pathological conditions, including inflammation, oxidative stress, cardiac disease, autoimmune system disorders, neurodegeneration, osteoporosis, depression, and cancer (El-Shitany and Eid, 2019[5]; He et al., 2020[10]). Though various in vivo and in vitro studies have revealed the anticancer effect of icariin, only a few systematic reviews have been published (Tan et al., 2016[23]). As there is a scarcity of studies reflecting the therapeutic role of icariin, the present letter highlights the beneficial effects of icariin in the treatment of different cancer. Further, it will provide a future direction to researchers and hints at developing safe and efficient anticancer drugs (Table 1(Tab. 1); References in Table 1: Alhakamy et al., 2020[3], 2021[2]; Alhakamy, 2021[1]; Cheng et al., 2019[4]; Fan et al., 2016[6]; Fang et al., 2019[7]; Gu et al., 2017[8]; Hao et al., 2019[9]; Huang et al., 2019[11]; Kim et al., 2020[12]; Lei et al., 2020[13]; Li et al., 2010[16], 2014[17], 2015[15], 2021[14]; Ma et al., 2014[18]; Shi et al., 2014[19]; Song et al., 2020[20]; Sun and Zhang, 2021[21]; Sun et al., 2016[22]; Tian et al., 2018[24]; Wang et al., 2010[28], 2015[29], 2017[25], 2019[26], 2020[27]; Wu et al., 2019[30]; Yang and Li, 2020[32]; Yang et al., 2015[31]; Zhang et al., 2013[33], 2014[34]). Ageal mucosa is normal.
Conflict of interest
The authors declare no conflict of interest.
References
1.
Alhakamy NA. Development and evaluation of icariin-loaded PLGA-PEG nanoparticles for potentiation the pro-apoptotic activity in pancreatic cancer cells. AAPS PharmSciTech. 2021;22 (8):252. doi: 10.1208/s12249-021-02111-w2.
Alhakamy NA, Badr Eldin SM, Alharbi WS, Alfaleh MA, Al-Hejaili OD, Aldawsari HM, et al. Development of an icariin-loaded bilosome-melittin formulation with improved anticancer activity against cancerous pancreatic cells. Pharmaceuticals (Basel). 2021;14:1309. doi: 10.3390/ph141213093.
Alhakamy NA, Fahmy FU, Badr Eldin SM, Ahmed OAA, Asfour HZ, Aldawsari HM, et al. Optimized icariin phytosomes exhibit enhanced cytotoxicity and apoptosis-inducing activities in ovarian cancer cells. Pharmaceutics. 2020;12(4):346. doi: 10.3390/pharmaceutics120403464.
Cheng X, Tan S, Duan F, Yuan Q, Li Q, Deng G. Icariin induces apoptosis by suppressing autophagy in tamoxifen-resistant breast cancer cell line MCF-7/TAM. Breast Cancer.2019;26:766-75. doi: 10.1007/s12282-019-00980-55.
El-Shitany NA, Eid B. Icariin modulates carrageenan-induced acute inflammation through HO-1/Nrf2 and NF-kB signaling pathways. Biomed Pharmacother. 2019;120:109567. doi: 10.1016/j.biopha.2019.1095676.
Fan C, Yang Y, Liu Y, Jiang S, Di S, Hu W, et al. Icariin displays anticancer activity against human esophageal cancer cells via regulating endoplasmic reticulum stress-mediated apoptotic signaling. Sci Rep. 2016;6:21145. doi: 10.1038/srep211457.
Fang L, Xu W, Kong D. Icariin inhibits cell proliferation, migration and invasion by down-regulation of microRNA-625-3p in thyroid cancer cells. Biomed Pharmacother. 2019;109:2456-63. doi: 10.1016/j.biopha.2018.04.0128.
Gu ZF, Zhang ZT, Wang JY, Xu BB. Icariin exerts inhibitory effects on the growth and metastasis of KYSE70 human esophageal carcinoma cells via PI3K/AKT and STAT3 pathways. Environ Toxicol Pharmacol. 2017;54:7-13. doi: 10.1016/j.etap.2017.06.0049.
Hao H, Zhang Q, Zhu H, Wen Y, Qiu D, Xiong J, et al. Icaritin promotes tumor T-cell infiltration and induces antitumor immunity in mice. Eur J Immunol. 2019;49:2235-44. doi: 10.1002/eji.20194822510.
He C, Wang Z, Shi J. Pharmacological effects of icariin. Adv Pharmacol. 2020;87:179-203. doi: 10.1016/bs.apha.2019.10.00411.
Huang S, Xie T, Liu W. Icariin inhibits the growth of human cervical cancer cells by inducing apoptosis and autophagy by targeting mTOR/PI3K/AKT signalling pathway. J BUON. 2019;24:990-612.
Kim B, Seo JH, Lee KY, Park B. Icariin sensitizes human colon cancer cells to TRAIL‑induced apoptosis via ERK‑mediated upregulation of death receptors. Int J Oncol. 2020;56:821-34. doi: 10.3892/ijo.2020.497013.
Lei K, Ma B, Shi P, Jin C, Ling T, Li L, et al. Icariin mitigates the growth and invasion ability of human oral squamous cell carcinoma via inhibiting toll-like receptor 4 and phosphorylation of NF-κB P65. OncoTargets Ther. 2020;13:299-307. doi: 10.2147/OTT.S21451414.
Li C, Yang S, Ma H, Ruan M, Fang L, Cheng J. Influence of icariin on inflammation, apoptosis, invasion, and tumor immunity in cervical cancer by reducing the TLR4/MyD88/NF-κB and Wnt/β-catenin pathways. Cancer Cell Int. 2021;21(1):206. doi: 10.1186/s12935-021-01910-215.
Li J, Jiang K, Zhao F. Icariin regulates the proliferation and apoptosis of human ovarian cancer cells through microRNA-21 by targeting PTEN, RECK and Bcl-2. Oncol Rep. 2015;33:2829-36. doi: 10.3892/or.2015.389116.
Li S, Dong P, Wang J, Zhang J, Gu J, Wu X, et al. Icariin, a natural flavonol glycoside, induces apoptosis in human hepatoma SMMC-7721 cells via a ROS/JNK-dependent mitochondrial pathway. Cancer Lett. 2010;298:222-30. doi: 10.1016/j.canlet.2010.07.00917.
Li W, Wang M, Wang L, Ji S, Zhang J. Zhang C. Icariin synergizes with arsenic trioxide to suppress human hepatocellular carcinoma. Cell Biochem Biophys. 2014;68:427-36. doi: 10.1007/s12013-013-9724-318.
Ma HR, Wang J, Chen YF, Chen H, Wang WS, Aisa HA. Icariin and icaritin stimulate the proliferation of SKBr3 cells through the GPER1-mediated modulation of the EGFR-MAPK signaling pathway. Int J Mol Med. 2014;33:1627-34. doi: 10.3892/ijmm.2014.172219.
Shi DB, Li XX, Zheng HT, Li DW, Cai GX, Peng JJ. et al. Icariin-mediated inhibition of NF-κB activity enhances the in vitro and in vivo antitumour effect of 5-fluorouracil in colorectal cancer. Cell Biochem Biophys. 2014;69:523-30. doi: 10.1007/s12013-014-9827-520.
Song L, Chen X, Mi L, Liu C, Zhu S, Yang T, et al. Icariin-induced inhibition of SIRT6/NF-κB triggers redox mediated apoptosis and enhances antitumor immunity in triple-negative breast cancer. Cancer Sci. 2020;111:4242-56. doi: 10.1111/cas.1464821.
Sun L, Zhang J. Icariin inhibits oral squamous cell carcinoma cell proliferation and induces apoptosis via inhibiting the NF-κB and PI3K/AKT pathways. Exp Ther Med. 2021;22(3):942. doi: 10.3892/etm.2021.1037422.
Sun Y, Sun XH, Fan WJ, Jiang XM, Li AW. Icariin induces S-phase arrest and apoptosis in medulloblastoma cells. Cell Mol Biol. 2016;62:123-923.
Tan HL, Chan KG, Pusparajah P, Saokaew S, Duangjai A, Lee LH, et al. Anti-cancer properties of the naturally occurring aphrodisiacs: Icariin and its derivatives. Front Pharmacol. 2016;7:191. doi: 10.3389/fphar.2016.0019124.
Tian M, Yang S, Yan X. Icariin reduces human colon carcinoma cell growth and metastasis by enhancing p53 activities. Braz J Med Biol Res. 2018;51:e7151-. doi: 10.1590/1414-431X20187151. 25.
Wang D, Xu W, Chen X, Han J, Yu L, Gao C, et al. Icariin induces cell differentiation and cell cycle arrest in mouse melanoma B16 cells via Erk1/2-p38-JNK-dependent pathway. Oncotarget. 2017;8:99504-13. doi: 10.18632/oncotarget.2011826.
Wang P, Zhang J, Xiong X, Yuan W, Qin S, Cao W, et al. Icariin suppresses cell cycle transition and cell migration in ovarian cancer cells. Oncol Rep. 2019;41:2321-8. doi: 10.3892/or.2019.698627.
Wang S, Gao J, Li Q, Ming W, Fu Y, Song L, et al. Study on the regulatory mechanism and experimental verification of icariin for the treatment of ovarian cancer based on network pharmacology. J Ethnopharmacol. 2020;262:113189. doi: 10.1016/j.jep.2020.11318928.
Wang Y, Dong H, Zhu M, Ou Y, Zhang J, Luo H, et al. Icariin exterts negative effects on human gastric cancer cell invasion and migration by vasodilator-stimulated phosphoprotein via Rac1 pathway. Eur J Pharmacol. 2010;635:40-8. doi: 10.1016/j.ejphar.2010.03.01729.
Wang Z, Zhang H, Dai L, Song T, Li P, Liu Y, et al. Arsenic trioxide and icariin show synergistic anti-leukemic activity. Cell Biochem Biophys. 2015;73:213-9. doi: 10.1007/s12013-015-0660-230.
Wu X, Kong W, Qi X, Wang S, Chen Y, Zhao Z, et al. Icariin induces apoptosis of human lung adenocarcinoma cells by activating the mitochondrial apoptotic pathway. Life Sci. 2019;239:116879. doi: 10.1016/j.lfs.2019.11687931.
Yang L, Wang Y, Guo H, Guo M. Synergistic anti-cancer effects of icariin and temozolomide in glioblastoma. Cell Biochem Biophys. 2015;71:1379-85. doi: 10.1007/s12013-014-0360-332.
Yang Y, Li G. Icariin inhibits proliferation, migration, and invasion of medulloblastoma DAOY cells by regulation of SPARC. Phytother Res. 2020;34:591-600. doi: 10.1002/ptr.654533.
Zhang DC, Liu JL, Ding YB, Xia JG, Chen GY. Icariin potentiates the antitumor activity of gemcitabine in gallbladder cancer by suppressing NF-κB. Acta Pharmacol Sin. 2013;34:301-8. doi: 10.1038/aps.2012.16234.
Zhang Y, Wei Y, Zhu Z, Gong W, Liu X, Hou Q, et al. Icariin enhances radiosensitivity of colorectal cancer cells by suppressing NF-κB activity. Cell Biochem Biophys. 2014;69:303-10. doi: 10.1007/s12013-013-9799-x
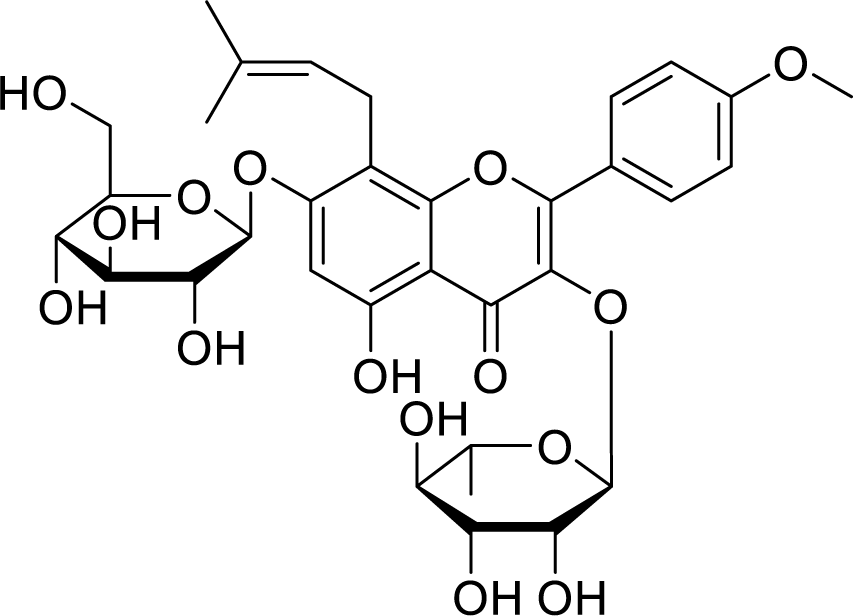
Figure 1: Chemical structure of icariin
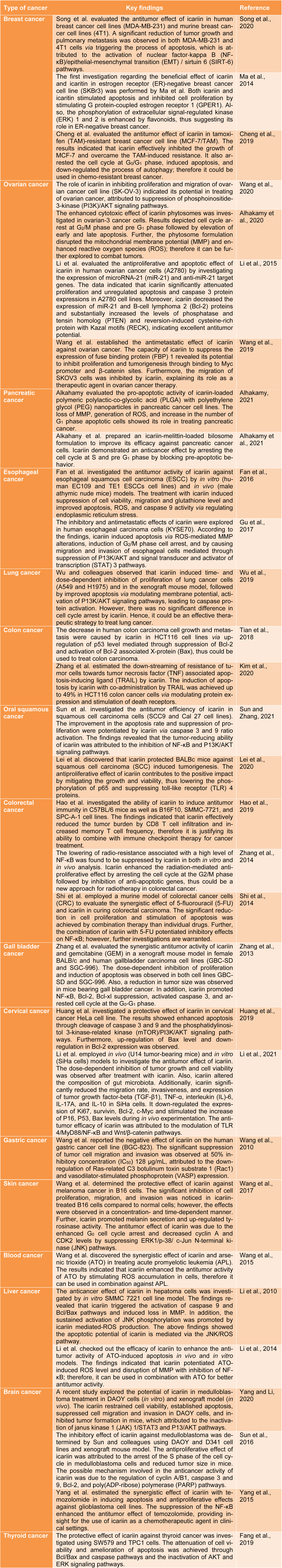
Table 1: An update on the protective effect of icariin in the treatment of different types of cancer

