Optimization of enzyme-linked immunosorbent assay kit protocol to detect trimethylamine N-oxide levels in humans
DOI:
https://doi.org/10.17179/excli2022-5617Keywords:
trimethylamine N-oxide, enzyme-linked immunosorbent assay, methods, investigative techniques, standardizationAbstract
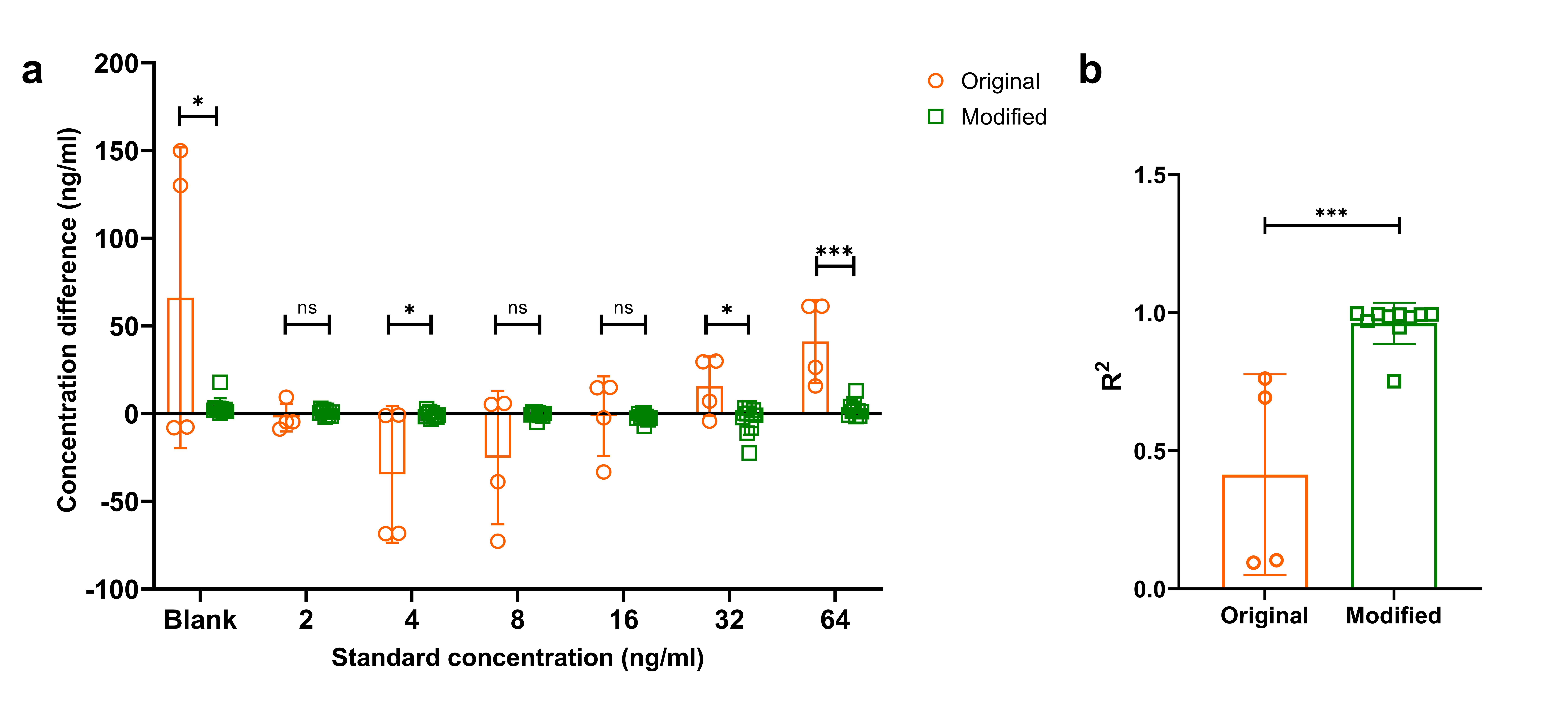
The serum level of trimethylamine N-oxide (TMAO), a gut microbiota metabolite associated with diabetes, cancer, inflammatory and neurological diseases, can be determined by the micro-enzyme-linked immunosorbent assay (ELISA) method. However, we had problems obtaining accurate standard curves with the original kit protocol from Bioassay Technology Laboratory. We aimed to acquire proper standard curves by modifying the kit protocol in this study. First, we evaluated the human TMAO ELISA kit protocols and other human ELISA kits. We maintained the incubation times longer and increased the wash cycle. Moreover, we incubated the standards containing biotinylated antibody in the wells alone. Then we washed the wells and added streptavidin-HRP for the second incubation step. The data of original and modified ELISA kit protocol were analyzed with Student's t-test. We measured higher absorbance with lower standard solution concentration in experiments that followed the original kit protocol. After investigating other human TMAO ELISA kits, we noticed that the SunRed Biotechnology Company and MyBioSource companies suggested similar protocols to the Bioassay Technology Laboratory company. The ELK Biotechnology ELISA protocol was different from others. However, since there is no biotinylated antibody in the standard solution in the ELK biotechnology kit, we changed some steps by examining other human ELISA protocols from different companies. After performing the modified protocol, we found that the absorbances of the standard solutions were consistent with their concentrations, and we obtained an accurate standard curve. Higher R2 values and lower absolute difference of standard concentrations were found in the modified kit protocol. The human TMAO ELISA protocol, which we modified in this study, will enable researchers to obtain more reliable results and prevent them from failing time and resources.
Downloads
Additional Files
Published
How to Cite
License
Copyright (c) 2023 Zinnet Sevval Aksoyalp, Betül Rabia Erdoğan, Saliha Aksun

This work is licensed under a Creative Commons Attribution 4.0 International License.
Authors who publish in this journal agree to the following terms:
- The authors keep the copyright and grant the journal the right of first publication under the terms of the Creative Commons Attribution license, CC BY 4.0. This licencse permits unrestricted use, distribution and reproduction in any medium, provided that the original work is properly cited.
- The use of general descriptive names, trade names, trademarks, and so forth in this publication, even if not specifically identified, does not imply that these names are not protected by the relevant laws and regulations.
- Because the advice and information in this journal are believed to be true and accurate at the time of publication, neither the authors, the editors, nor the publisher accept any legal responsibility for any errors or omissions presented in the publication. The publisher makes no guarantee, express or implied, with respect to the material contained herein.
- The authors can enter into additional contracts for the non-exclusive distribution of the journal's published version by citing the initial publication in this journal (e.g. publishing in an institutional repository or in a book).





