GLUL gene knockdown and restricted glucose level show synergistic inhibitory effect on the luminal subtype breast cancer MCF7 cells’ proliferation and metastasis
DOI:
https://doi.org/10.17179/excli2023-6287Keywords:
cancer metabolism, glutamine synthetase, glycolysis, luminal breast cancer, MCF7 cell lineAbstract
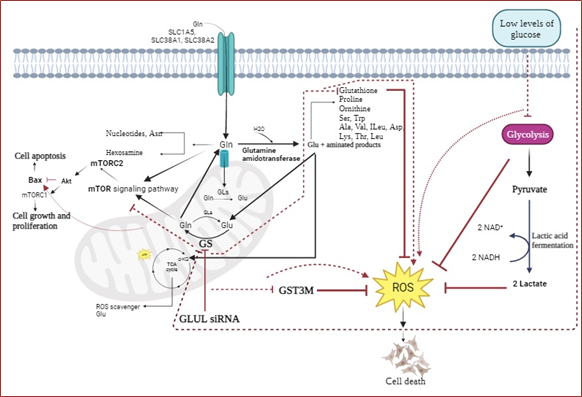
The glutamine synthetase path is one of the most important metabolic pathways in luminal breast cancer cells, which plays a critical role in supplying glutamine as an intermediate in the biosynthesis of amino acids and nucleotides. On the other hand, glycolysis and its dominant substrate, glucose, are the most critical players in cancer metabolism. Accordingly, targeting these two critical paths might be more efficient in luminal-type breast cancer treatment. MCF7 cells were cultivated in media containing 4.5, 2, and 1 g/L glucose to study its effects on GLUL (Glutamate Ammonia Ligase) expression. Followingly, high and low glucose cell cultures were transfected with 220 pM of siGLUL and incubated for 48 h at 37 ºC. The cell cycle progression and apoptosis were monitored and assessed by flow cytometry. Expression of GLUL, known as glutamine synthetase, was evaluated in mRNA and protein levels by qRT-PCR and western blotting, respectively. To examine the migration and invasion capacity of studied cells exploited from wound healing assay and subsequent expression studies of glutathione-S-transferase Mu3 (GSTM3) and alfa-enolase (ENO1). Expression of GLUL significantly decreased in cells cultured at lower glucose levels compared to those at higher glucose levels. siRNA-mediated knockdown of GLUL expression in low glucose cultures significantly reduced growth, proliferation, migration, and invasion of the MCF7 cells and enhanced their apoptosis compared to the controls. Based on the results, GLUL suppression down-regulated GSTM3, a main detoxifying enzyme, and up-regulated Bax. According to the role of glycolysis as a ROS suppressor, decreased amounts of glucose could be associated with increased ROS; it can be considered an efficient involved mechanism in this study. Also, increased expression of Bax could be attributable to mTOR/AKT inhibition following GLUL repression. In conclusion, utilizing GLUL and glycolysis inhibitors might be a more effective strategy in luminal-type breast cancer therapy.
Downloads
Additional Files
Published
How to Cite
License
Copyright (c) 2023 Arezu Karimpur Zahmatkesh, Mohammad Khalaj-Kondori, Mohammad Ali Hosseinpour Feizi, Behzad Baradaran

This work is licensed under a Creative Commons Attribution 4.0 International License.
Authors who publish in this journal agree to the following terms:
- The authors keep the copyright and grant the journal the right of first publication under the terms of the Creative Commons Attribution license, CC BY 4.0. This licencse permits unrestricted use, distribution and reproduction in any medium, provided that the original work is properly cited.
- The use of general descriptive names, trade names, trademarks, and so forth in this publication, even if not specifically identified, does not imply that these names are not protected by the relevant laws and regulations.
- Because the advice and information in this journal are believed to be true and accurate at the time of publication, neither the authors, the editors, nor the publisher accept any legal responsibility for any errors or omissions presented in the publication. The publisher makes no guarantee, express or implied, with respect to the material contained herein.
- The authors can enter into additional contracts for the non-exclusive distribution of the journal's published version by citing the initial publication in this journal (e.g. publishing in an institutional repository or in a book).





